Can A Ka Value Be Negative For acids like HCl HBr and HI their pKa values are negative What do the negative numbers mean and how is it possible to get negative pKa values pKa log10 Ka The log10 of a
Yes pKa and pKb values can be negative The pKa and pKb are the negative logarithms of the acid dissociation constant Ka and the base dissociation constant Kb PKa is the negative log base ten of the Ka value acid dissociation constant It measures the strength of an acid The lower the value of pKa the stronger the acid and the greater its ability to donate its protons
Can A Ka Value Be Negative

Can A Ka Value Be Negative
https://i.ytimg.com/vi/I3rWS73IJlo/maxresdefault.jpg

Question Video Writing An Equation For The Acid Dissociation Constant
https://media.nagwa.com/692148986282/en/thumbnail_l.jpeg
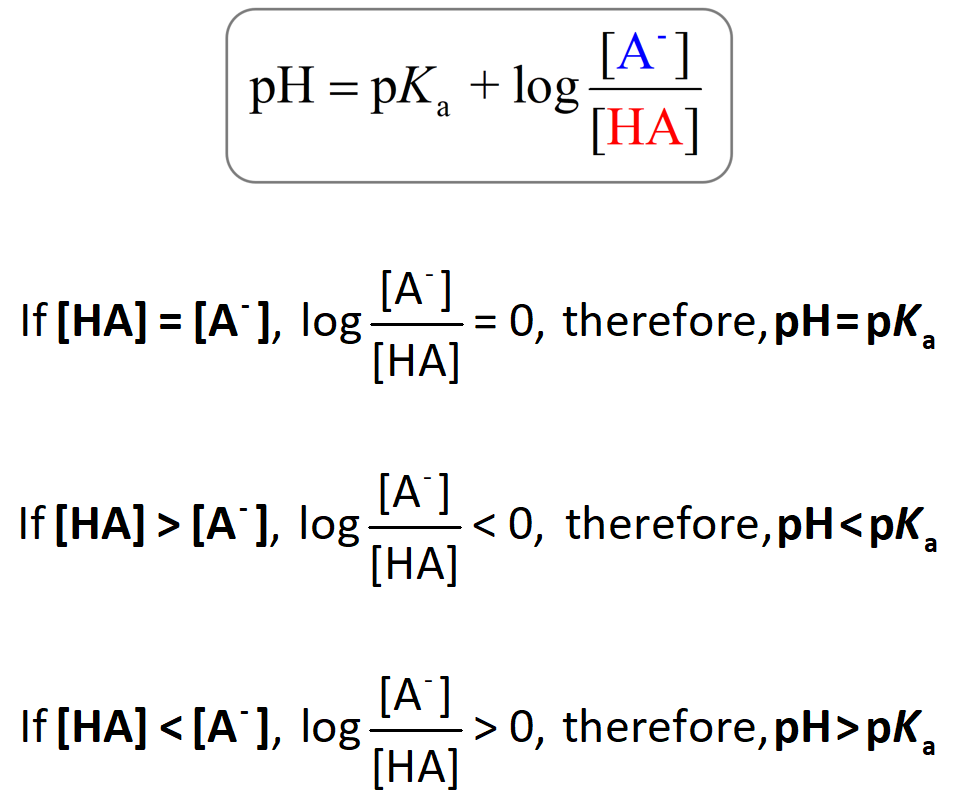
PH And PKa Relationship Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/06/pH-and-pKa-Relationship.png
As such a higher Pka is indicative of a more acidic compound the p Ka of the same compound is equivalent to taking the log pKa which is how one could get negative Whenever you see a p in front of a value like pH pKa and pKb it means you re dealing with a negative log of the value following the p For example pKa is the negative log of Ka Because of the way the log function
PKa is the negative logarithm of the Ka value p K a log K a pK a log K a pKa provides a more convenient way to express the strength of an acid The lower A p in front of a value also indicates the log of the value So pH is the negative log of hydrogen ion concentration while pKa is the negative log of the Ka value The capital letter K stands for a constant In this case it refers
More picture related to Can A Ka Value Be Negative

What Is PKa In Chemistry Acid Dissociation Constant
https://sciencenotes.org/wp-content/uploads/2021/10/What-Is-pKa-in-Chemistry.png

no R dze Uzavreli Zmluvu Terminal Value Calculation Palica Kent Vypr zdni
https://wsp-blog-images.s3.amazonaws.com/wsp_template_library/Terminal-Value-Calculator.jpg

Water Ka Value Best 23 Answer Barkmanoil
https://i.ytimg.com/vi/IQ6gHQAg_Nk/maxresdefault.jpg
Can Ka be Negative Explore the possibility of negative Ka values and the implications for acidic solutions Understand the scenarios where negative Ka values may arise How Does Ka can t be negative otherwise a reaction component has a negative concentration Furthermore it would be infinitely large and not less than one as the numerator products is far greater
Can pKa be negative Yes pKa can be negative for very strong acids with a Ka value greater than 1 6 What is the relationship between pKa and Ka The relationship is PKa is the negative base 10 logarithm of Ka acid dissociation constant of a solution pKa is the characteristic of a particular compound and it tells about how readily the
:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)
Ka Values And Acids
https://www.thoughtco.com/thmb/K046VWvlGuvdE0hUANDAMVDJLJ0=/1500x0/filters:no_upscale():max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png

What Is PKa In Chemistry Acid Dissociation Constant
https://sciencenotes.org/wp-content/uploads/2021/10/What-Is-pKa-in-Chemistry-1024x683.png

https://www.reddit.com › chemistry › comments › ...
For acids like HCl HBr and HI their pKa values are negative What do the negative numbers mean and how is it possible to get negative pKa values pKa log10 Ka The log10 of a
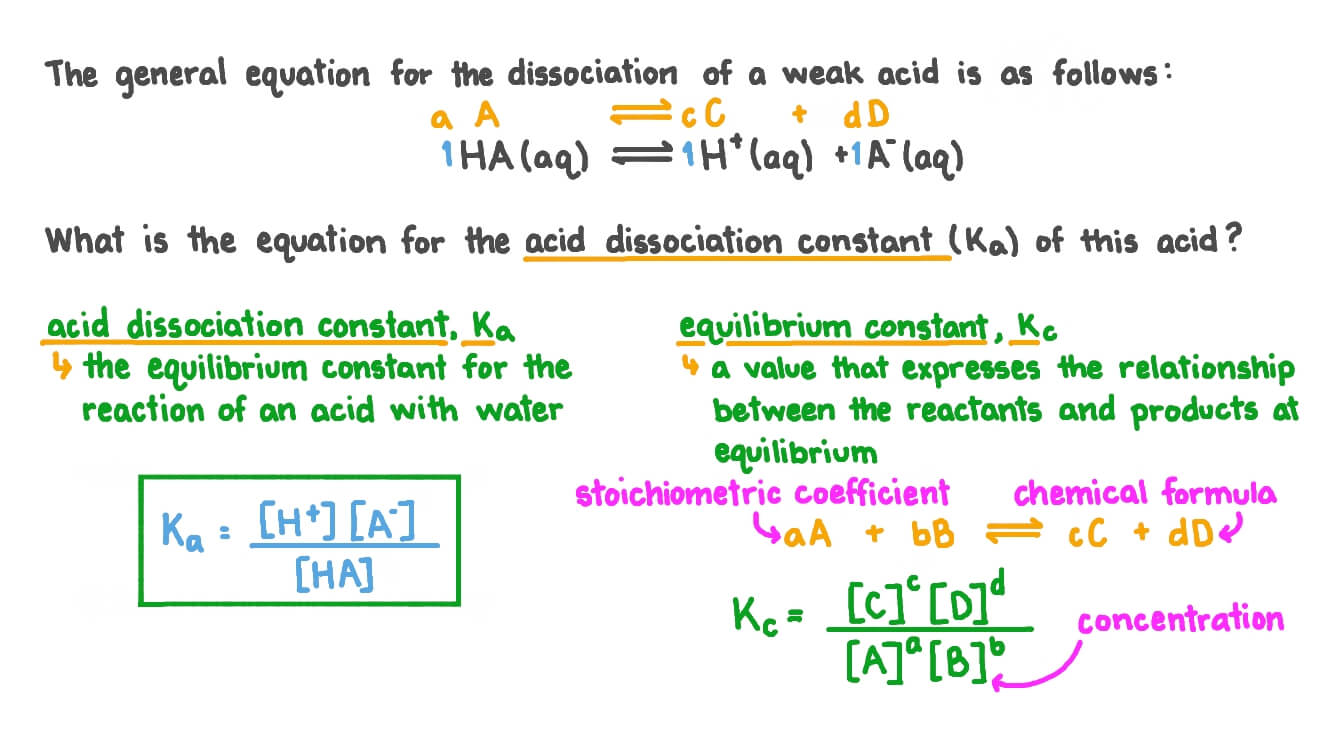
https://lavelle.chem.ucla.edu › forum › viewtopic.php
Yes pKa and pKb values can be negative The pKa and pKb are the negative logarithms of the acid dissociation constant Ka and the base dissociation constant Kb

How To Get Pka From Ka Peterson Hinse1964
:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)
Ka Values And Acids

Ph From Ka And Molarity Calculator

Calculate Ka Of Acetic Acid If Its 0 05 M Solution Has Molar

Titration Diagram

How To Calculate PH And Ka EQUILIBRIUM CONSTANT From PERCENT

How To Calculate PH And Ka EQUILIBRIUM CONSTANT From PERCENT

Weak Acids PH Ka And PKa YouTube

Fair Value Gap Trading Bank2home

11 Use PH And Ka To Find Initial Concentration YouTube
Can A Ka Value Be Negative - A higher Ka value indicates a stronger acid meaning it donates protons more easily Weak acids have smaller Ka values The pKa is the negative logarithm of the Ka value