Clinical Data Management Specialist Salary Everest Clinical trials are a type of research that studies new tests and treatments and evaluates their effects on human health outcomes People volunteer to take part in clinical
The Guidance for Best Practices for Clinical Trials has been developed in response to the 2022 World Health Assembly resolution WHA75 8 on strengthening clinical trials This guidance What is a clinical trial For the purposes of registration a clinical trial is any research study that prospectively assigns human participants or groups of humans to one or
Clinical Data Management Specialist Salary Everest

Clinical Data Management Specialist Salary Everest
https://i.ytimg.com/vi/V7_cQmlrM44/maxresdefault.jpg

IT Specialist Job Description Information Technology Specialist IT
https://i.ytimg.com/vi/vsjZvSbFtWw/maxresdefault.jpg

What Is Clinical Data Management Clinical Data Management System
https://i.ytimg.com/vi/AbXwsttNlWU/maxresdefault.jpg
The WHO Clinical Registry is a free web based platform that helps health facilities systematically collect analyze and use clinical data to improve emergency care outcomes It is housed on Clinical trials in children The mission of the WHO International Clinical Trials Registry Platform is to ensure that a complete view of research is accessible to all those involved in health care
The 70th World Health Assembly called for the development of guidance on sepsis prevention and management to support Members States in Improving the prevention WHO develops up to date technical guidance for clinical management of influenza patients focused on antiviral and adjunct therapeutics
More picture related to Clinical Data Management Specialist Salary Everest

What Is Clinical Data Management Clinical Data Management YouTube
https://i.ytimg.com/vi/2eRqrNVfzPc/maxresdefault.jpg
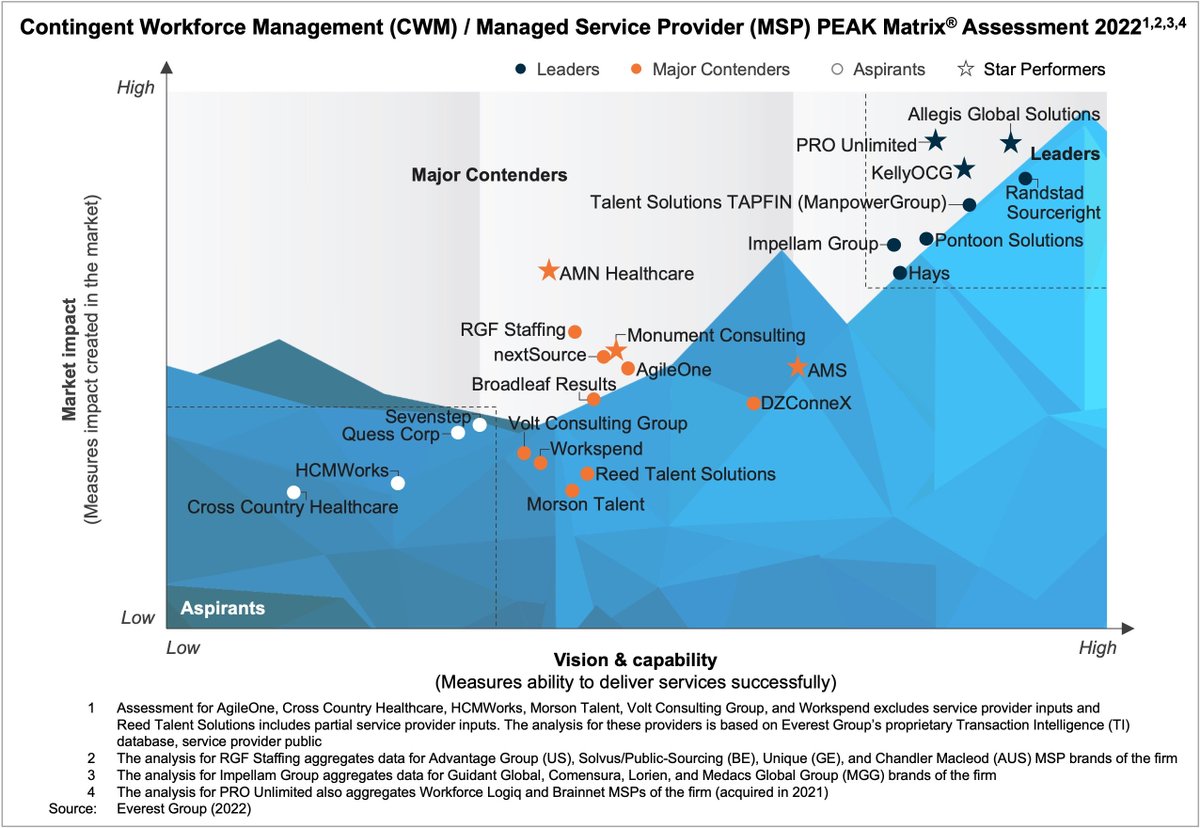
Everest Group On Twitter Congratulations AllegisGlobal Randstad SR
https://pbs.twimg.com/media/FdlaTLBWYAIcvyk.jpg

Data Entry Specialist Job Description
https://cdn.feedingtrends.com/article-images/Get_Paid_Stock_com_63d7cef75258f_0402b89abc.jpg
A clinical trials registry is the entity that houses the register and is responsible for ensuring the completeness and accuracy of the information it contains and that the registered Advising WHO on the draft Clinical trial unit maturity framework to support benchmarking of infrastructure capabilities and capacities of institutions in conducting clinical
[desc-10] [desc-11]
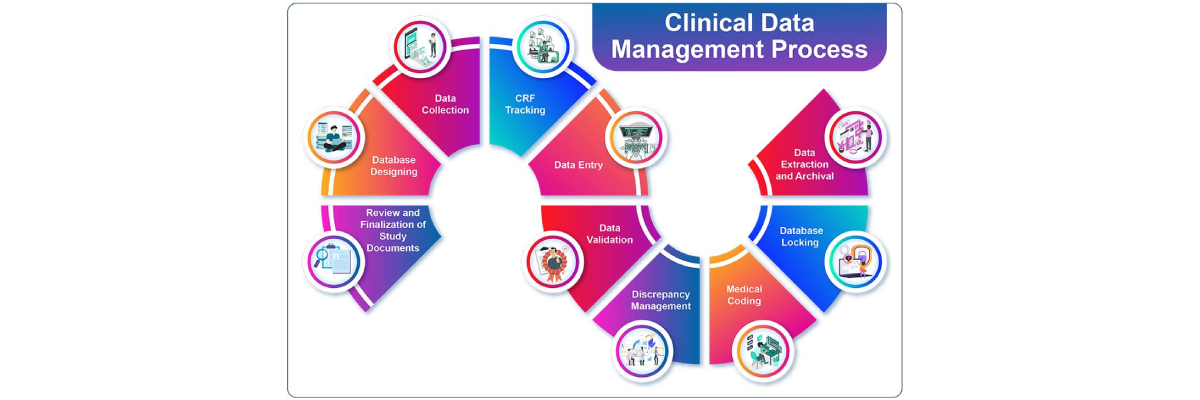
PharbioMed
https://pharbiomed.com/images/clinical_data_management_image.png

Arnex
https://www.arnexsolution.com/img/logo-2.png

https://www.who.int › health-topics › clinical-trials
Clinical trials are a type of research that studies new tests and treatments and evaluates their effects on human health outcomes People volunteer to take part in clinical

https://www.who.int › ... › guidance-for-best-practices-for-clinical-trials
The Guidance for Best Practices for Clinical Trials has been developed in response to the 2022 World Health Assembly resolution WHA75 8 on strengthening clinical trials This guidance

What We Do Health Data Acumen

PharbioMed

What We Do Health Data Acumen
Sample Management Precision Medicine Scientific Research Service Platform
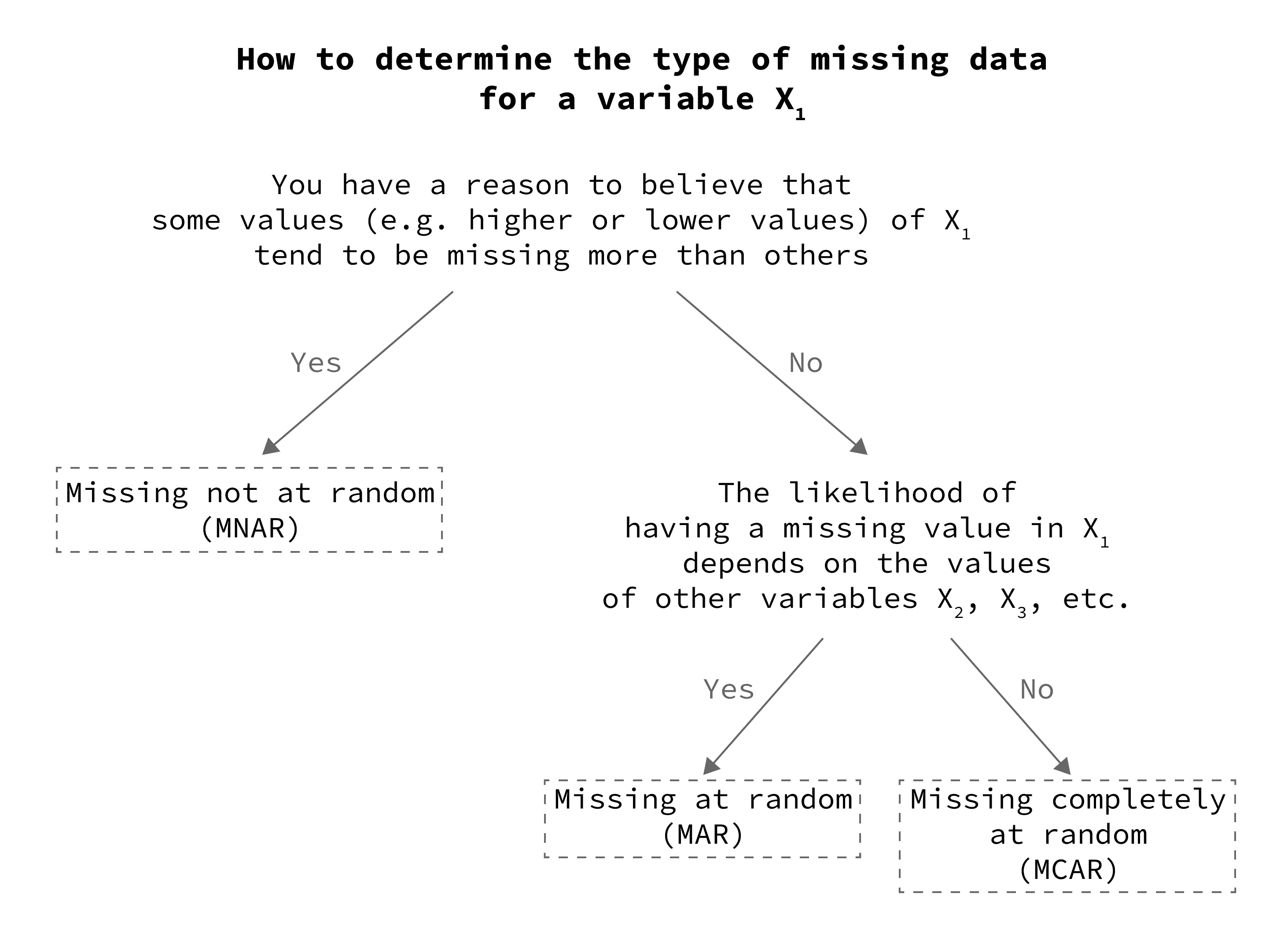
How To Handle Missing Data In Practice Guide For Beginners

Healthcare Analytics And Clinical Data Management Onebridge

Healthcare Analytics And Clinical Data Management Onebridge

Clinical Data Management CDM Clininet in

Order Management Specialist Salary Job Description

Clinical Informatics Specialist Salary Career Overview USC MPH
Clinical Data Management Specialist Salary Everest - WHO develops up to date technical guidance for clinical management of influenza patients focused on antiviral and adjunct therapeutics