Current Good Manufacturing Practices Are Covered In Which Document Current Good Manufacturing Practice Hazard Analysis and Risk Based Preventive Controls for Human Food 117 1 117 475
The Food and Drug Administration establishes maximum levels for these defects in foods produced under current good manufacturing practice and uses these levels in deciding The applicable requirements are established in our regulations entitled Current Good Manufacturing Practice Hazard Analysis and Risk Based Preventive Controls for Food
Current Good Manufacturing Practices Are Covered In Which Document
Current Good Manufacturing Practices Are Covered In Which Document
https://www.advotics.com/wp-content/uploads/2023/02/Good-Manufacturing-Practices-Simak-Pengertian-Indikator-dan-Manfaat-01.png

TUV SUD Codex GMP Certificate Certificates HSIEHS BIOTECH
https://www.hsbiotech.com/upfile/2016/02/20160205173248_585.jpg
Good Manufacturing Practices ISO CONSULTANTS
http://www.sgqinnovations.com/wp-content/uploads/2016/09/AAEAAQAAAAAAAAMaAAAAJGE5Y2QzOTRmLTk0MDMtNGE5YS1hMzVhLTk3MDg5Y2NlZmM2ZQ.png
All persons working in direct contact with food food contact surfaces and food packaging materials must conform to hygienic practices while on duty to the extent necessary to protect Good Manufacturing Practices GMP also referred to as cGMP or current Good Manufacturing Practice is the aspect of quality assurance that ensures that medicinal products are consistently produced and controlled to the quality
This report is based on the assessment against the regulations and requirements set out in the Good Manufacturing Practices GMP guidance document It is considered a self Current version of the Guide to Good Manufacturing Practice Rules Governing Medicinal Products in the European Community Volume IV 2 For the United States Copies
More picture related to Current Good Manufacturing Practices Are Covered In Which Document

Company Background Optipharm Therapeutics
https://optipharm.co.ke/wp-content/uploads/2024/08/Optipharm-Logo-white.fw_-2-1536x1131.png

Revised Annex 1 And The Focus On Microbial Control Strategies
https://resilience.com/hubfs/AdobeStock_731212541.jpeg

Sustainable Products Definition Arena
https://www.arenasolutions.com/wp-content/uploads/what-are-sustainable-products-768x768.png
CGMP or Current Good Manufacturing Practices encompasses a broad range of principles to ensure products are consistently produced and controlled according to quality standards These principles cover everything As part of the Current Good Manufacturing Practices CGMP initiative announced in August of 2002 and to help FDA be more transparent with CGMP policy we have
GMP covers all aspects of the manufacturing process defined manufacturing process validated critical manufacturing steps suitable premises storage transport qualified and trained 21 CFR Parts 210 211 are applicable to pharmaceutical manufacturing 21 CFR Part 210 CURRENT GOOD MANUFACTURING PRACTICE IN MANUFACTURING PROCESSING

Regcon NovaHealth
https://novahealth.com.pk/wp-content/uploads/2024/07/regcon-shape-updated.png

Current Good Manufacturing Practices CGMP NCBioNetwork
https://www.ncbionetwork.org/sites/default/files/styles/1280x720/public/1280x720-images/cgmp_preview.jpg?itok=l24WdU6c

https://www.ecfr.gov › current › chapter-I › subchapter-B
Current Good Manufacturing Practice Hazard Analysis and Risk Based Preventive Controls for Human Food 117 1 117 475

https://www.ecfr.gov › current › chapter-I › subchapter-B
The Food and Drug Administration establishes maximum levels for these defects in foods produced under current good manufacturing practice and uses these levels in deciding

CGMP For Biopharmaceutical Drug Products Live Online Biotility

Regcon NovaHealth

Good Manufacturing Practice Poster Food Safety Works

Slimax NovaHealth

Good Manufacturing Practices Abstract Iso 22716 Certified Stock Vector
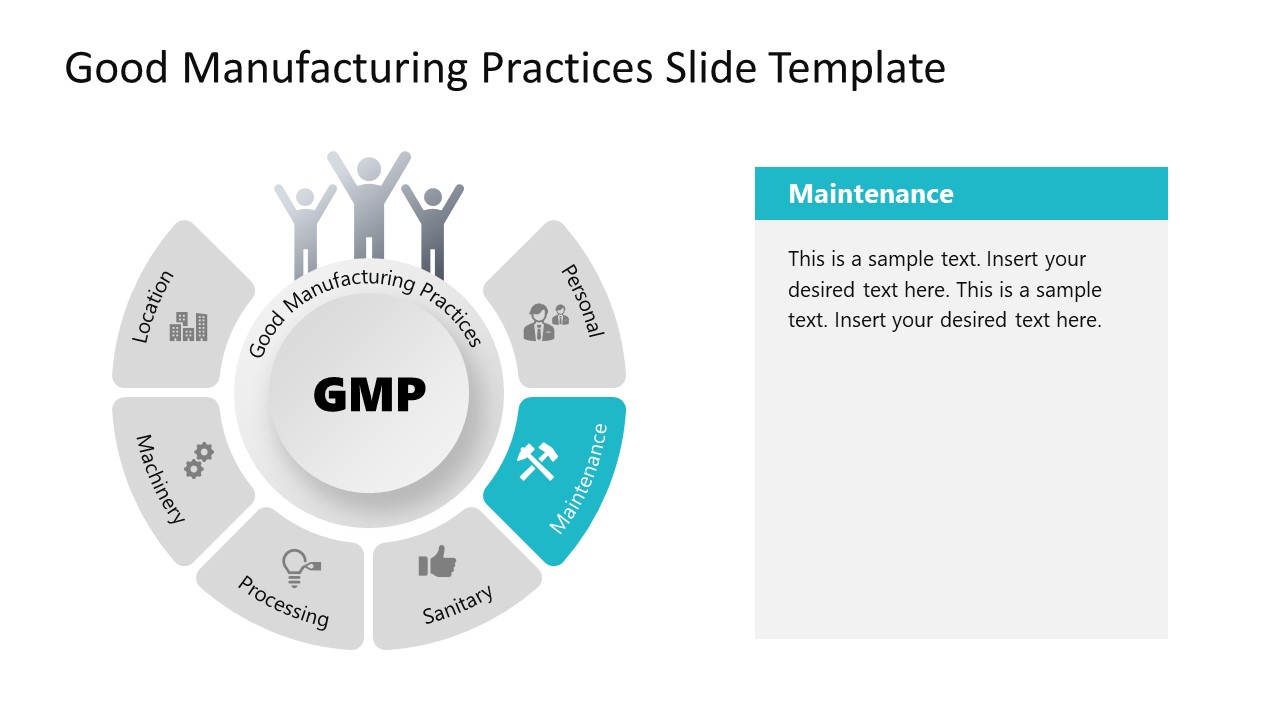
Good Manufacturing Practices PowerPoint Template

Good Manufacturing Practices PowerPoint Template

Current Good Manufacturing Practice CGMP Definition Arena

Good Manufacturing Practices Abstract Iso 22716 Certified Stock Vector

Good Manufacturing Practices GMP
Current Good Manufacturing Practices Are Covered In Which Document - CGMP guidelines cover every aspect of production from facility design to staff training equipment calibration quality control and record keeping These practices help