Does Heat Affect Activation Energy The activation energy E a labeled Delta G ddagger in Figure 2 is the energy difference between the reactants and the activated complex also known as transition
Being a measure of energy temperature can be used as one of what could be several energy input paths that help a reaction matrix reach its activation energy Higher or lower temperature raises and lowers the further No the temperature does not affect activation energy A catalyst is needed to lower the activating energy What is the significance of calculating activation energy
Does Heat Affect Activation Energy

Does Heat Affect Activation Energy
https://i.ytimg.com/vi/QjcMgk45sOQ/maxresdefault.jpg
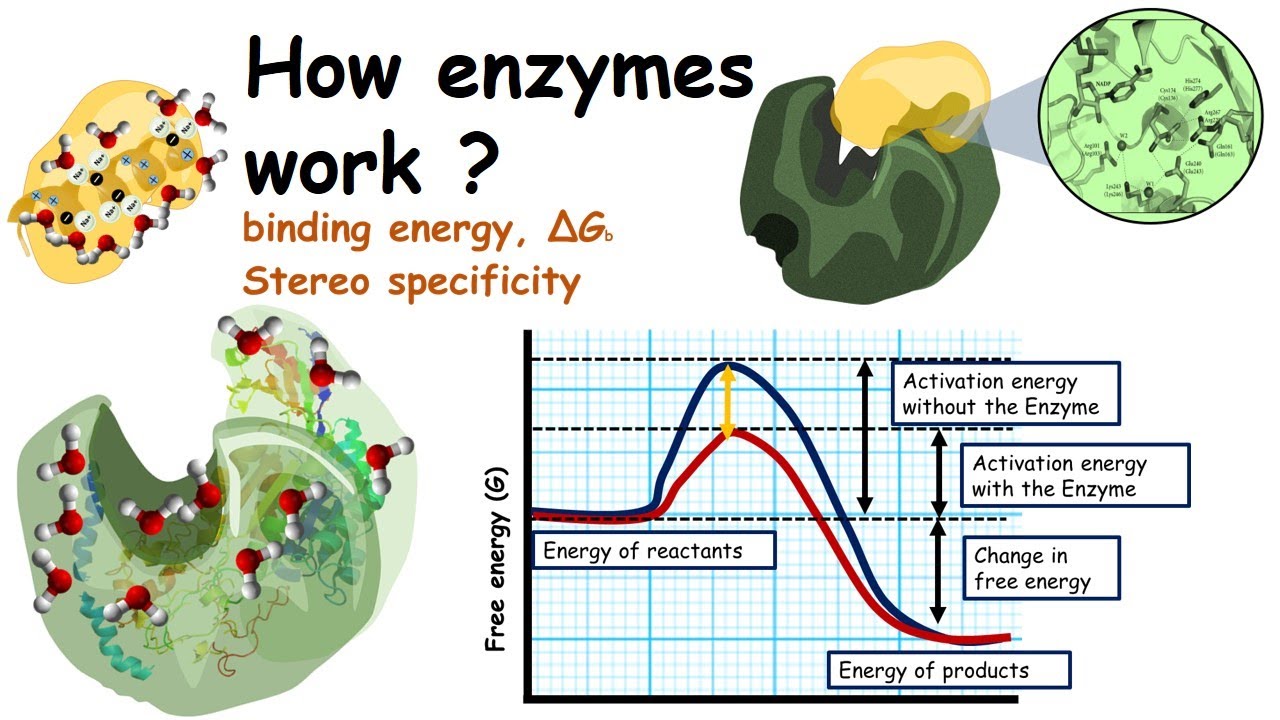
How Enzymes Work Lowering Of Activation Energy By Enzymes And Binding
https://i.ytimg.com/vi/wiIUS2LDCl8/maxresdefault.jpg
Activation Energy Arrhenius Equation
http://cimg1.ck12.org/datastreams/f-d:df0a2687d885c997ec852a60b09181c51b0a234ada9136e0288d4e8c%2BIMAGE%2BIMAGE.1
Activation energy is the minimum energy with which reactants must collide in order for a reaction to occur The source of the activation energy needed to push reactions forward is typically Activation energy can be thought of as the magnitude of the potential barrier sometimes called the energy barrier separating minima of the potential energy surface pertaining to the initial and final thermodynamic state
The effect of increasing collision frequency on the rate of the reaction is very minor The important effect is quite different The key importance of activation energy Collisions only result in a reaction if the particles collide with enough Higher temperature means higher kinetic energy but it does not mean the potential energy barrier is lower It means there is higher probability to overcome it leading to a faster reaction There is a famour Arrhenius
More picture related to Does Heat Affect Activation Energy

Boltzmann Distribution Curves A Level ChemistryStudent
https://www.chemistrystudent.com/images/ASPhysical/kinetics/maxwell-boltzmann2.png
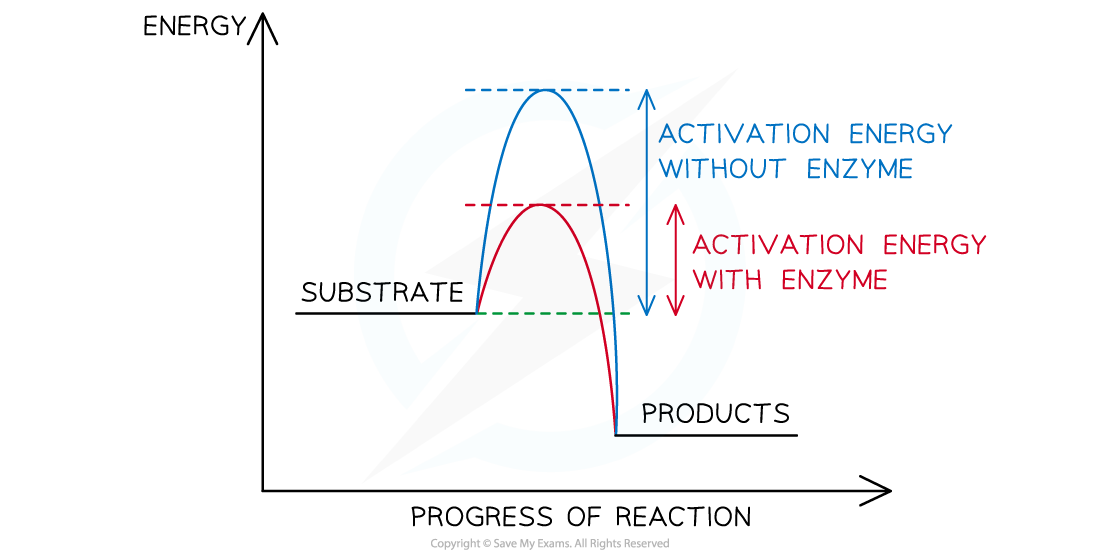
IB DP Biology HL 8 1 1 Metabolic Pathways
https://oss.linstitute.net/wechatimg/2022/07/enzymes-activation-energy-1.png

Enzymes Activation Energy Cartoon
https://i.ytimg.com/vi/ueup2PTkFW8/maxresdefault.jpg
Thermal energy in the form of heat is supplied to the molecules increasing their momentum and kinetic energy As the temperature increases the molecules move faster and collide frequently If the temperature increases further Apply the Arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics represented by the pre exponential factor A Describe how collision frequencies and
Activation energy is the minimum energy required for reactants to successfully react and turn in to products In equilibrium reactions there is an activation energy for both the forward and Reactants often get activation energy from heat but sometimes energy comes from light or energy released by other chemical reactions For spontaneous reactions the ambient
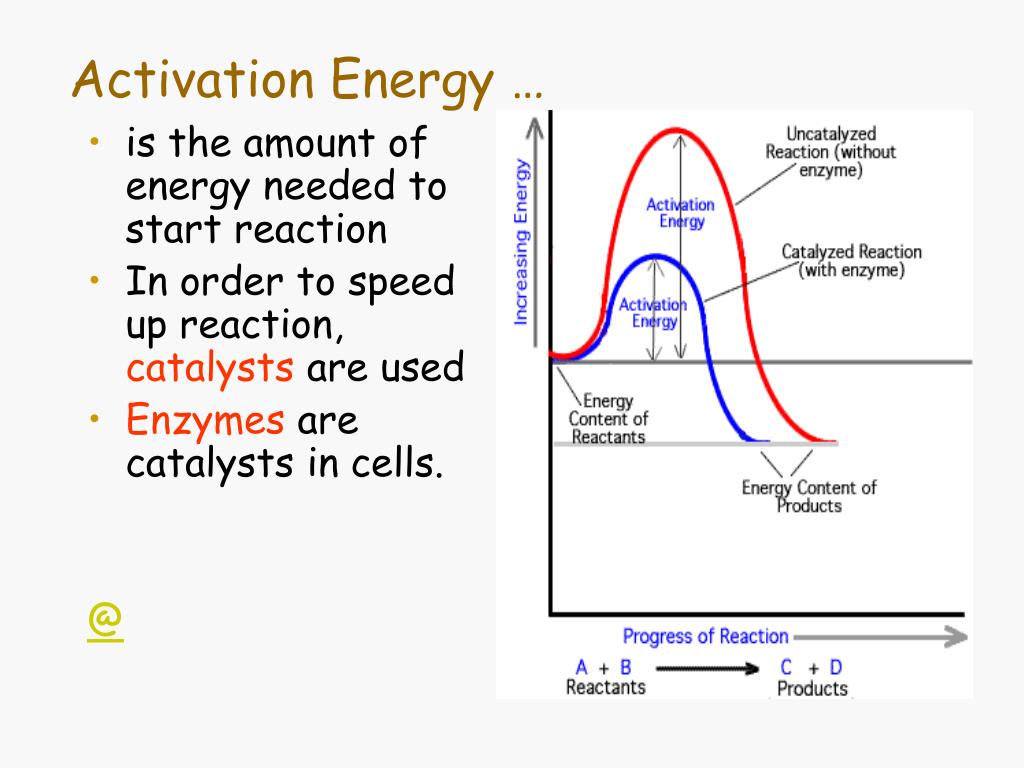
Enzymes Activation Energy
https://image2.slideserve.com/4880453/activation-energy-l.jpg

Activation Energy Examples
https://cdn1.byjus.com/wp-content/uploads/2023/02/Activation-Energy-3.png

https://chem.libretexts.org › Bookshelves › Physical...
The activation energy E a labeled Delta G ddagger in Figure 2 is the energy difference between the reactants and the activated complex also known as transition

https://www.sciencing.com
Being a measure of energy temperature can be used as one of what could be several energy input paths that help a reaction matrix reach its activation energy Higher or lower temperature raises and lowers the further

Enzyme Activation Energy

Enzymes Activation Energy

Activation Energy Catalyst
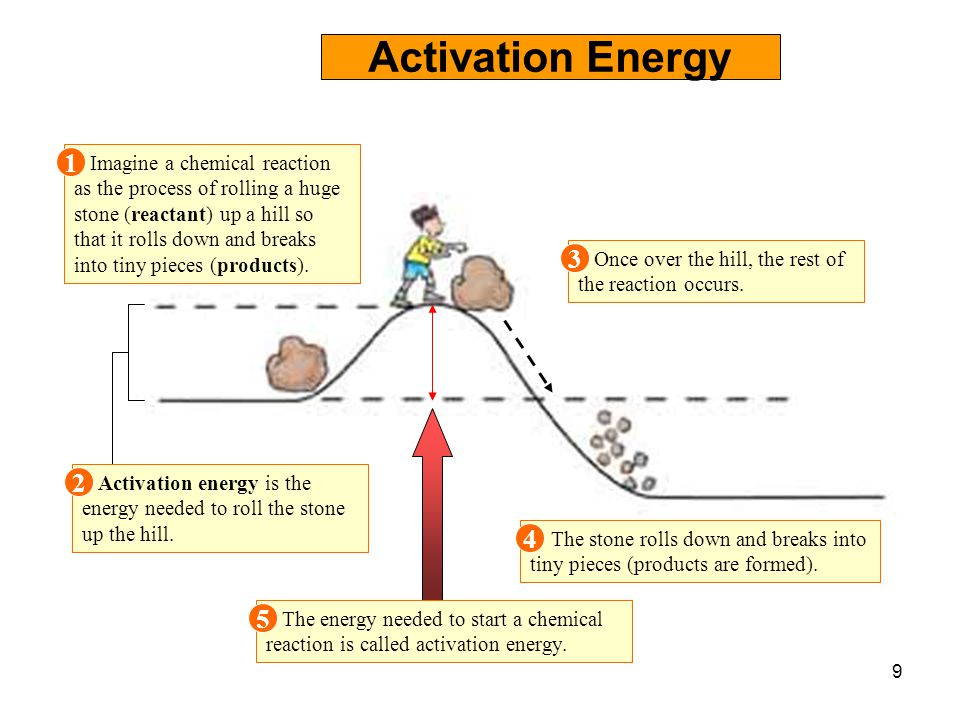
Activation Energy Examples

Illustrate Graphically The Effect Of A Catalyst On Rate Of A Reaction
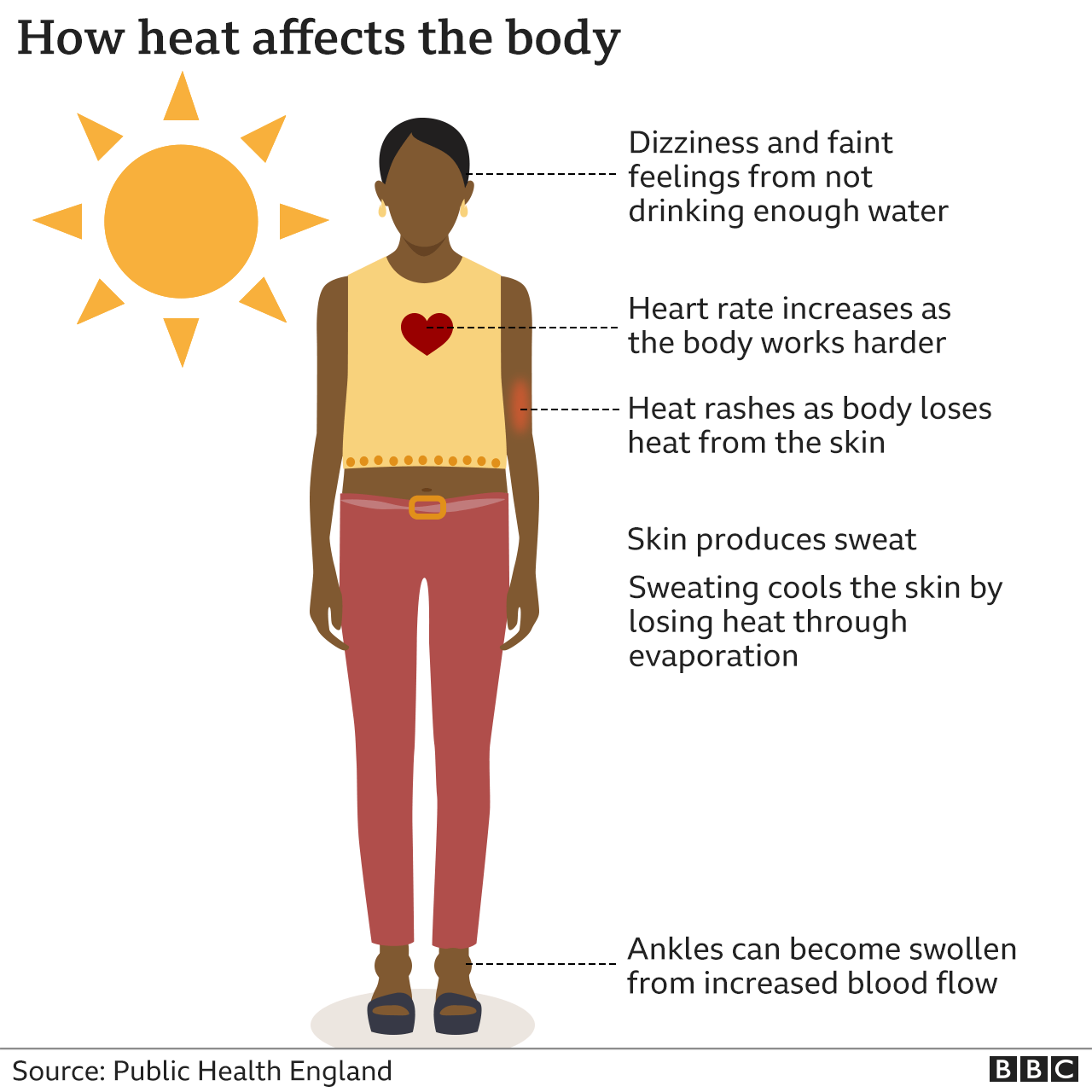
How Do Heat And Cold health Alerts Work BBC News

How Do Heat And Cold health Alerts Work BBC News
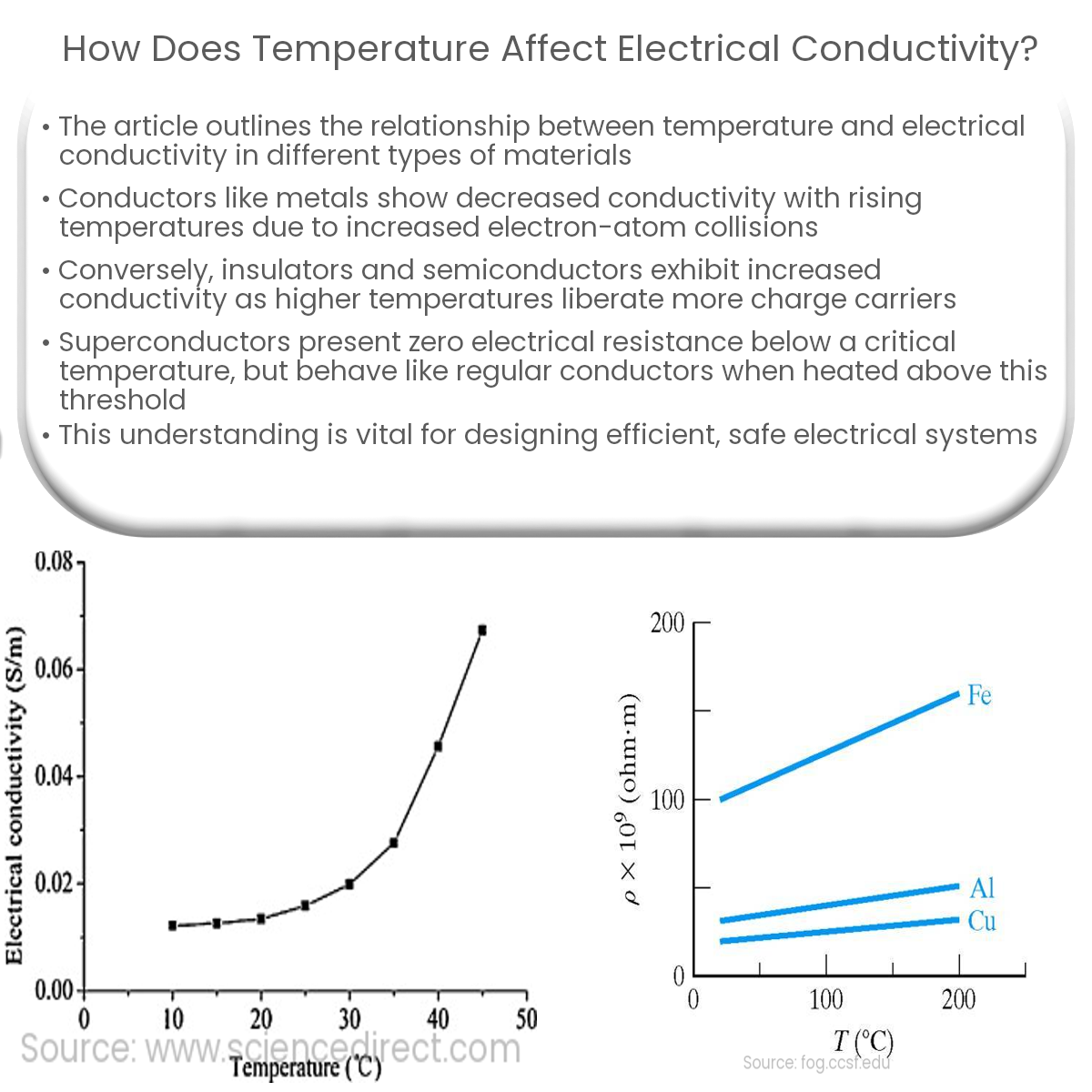
How Does Temperature Affect Electrical Conductivity

Potential Energy Diagram Labeled
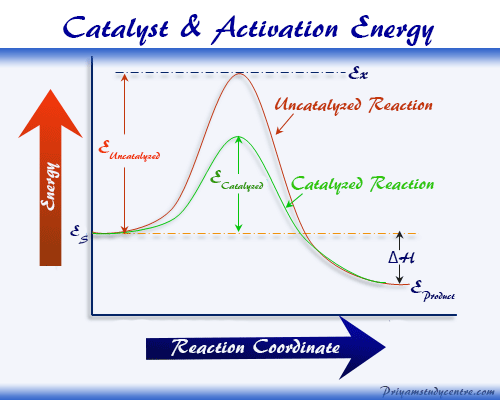
Chemical Catalyst Definition Reaction Types And Examples
Does Heat Affect Activation Energy - Heat energy the total bond energy of reactants or products in a chemical reaction speeds up the motion of molecules increasing the frequency and force with which they collide