Half Life Formula The formulas for half life are t ln2 and t tln2 ln N 0 N t The equation for exponential decay is 1 N t N 0e t where N 0 is the initial quantity N t is the quantity at time t is the exponential decay constant We can solve this for 2 1 tln N 0 N t And the formulas for half life t are 3 t ln2 and 4 t tln2 ln N 0 N t If you know the
Half life is defined as the time after which half of a sample of a radioactive material will have decayed In other words if you start with 1 kg of material with a half life of 1 year then after 1 year you will have 500g After another year you will have half of that or 250 g After another year you will have 125 g and so on Exponential decay is usually represented by an exponential function of time with base e and a negative exponent increasing in absolute value as the time passes F t A e K t where K is a positive number characterizing the speed of decay Obviously this function is descending from some initial value at t 0 down to zero as time increases towards infinity For
Half Life Formula

Half Life Formula
http://www.wikihow.com/images/5/52/Calculate-Half-Life-Step-6.jpg

Master The HALF LIFE Equation Derivation Dive Deeper
https://warreninstitute.org/wp-content/uploads/understanding-the-half-life-equation-derivation.png

Half Life Formula And Calculation Understanding Radioactive Decay
https://i.ytimg.com/vi/Jle-3D7oI6s/maxresdefault.jpg
If we start from an initial concentration of A it is A 0 Then it approaches a final concentration A that is half in quantity Therefore the first half life is written as A 1 2 A 0 To find further half lives keep halving the concentration n times Use the radioactive decay half life formula The radioactive decay half life formula states that N t N 0 1 2 t t 1 2 where N t is the final amount of substance N 0 is the initial amount of a substance t is the time usually in years or seconds t 1 2 is the half life of the substance To solve for half life of a substance rearrange the formula in terms of t 1 2
You could use this formula Where Th half life M the beginning amount M the ending amount One example of how to use the equation One of the Nuclides in spent nuclear fuel is U 234 an alpha emitter with a half life of 2 44 x10 5 years See explanation The half life of a chemical reaction regardless of its order is simply the time needed for half of an initial concentration of a reactant to be consumed by the reaction Now a first order reaction is characterized by the fact that the rate of the reaction depends linearly on the concentration of one reactant For a first order reaction A
More picture related to Half Life Formula
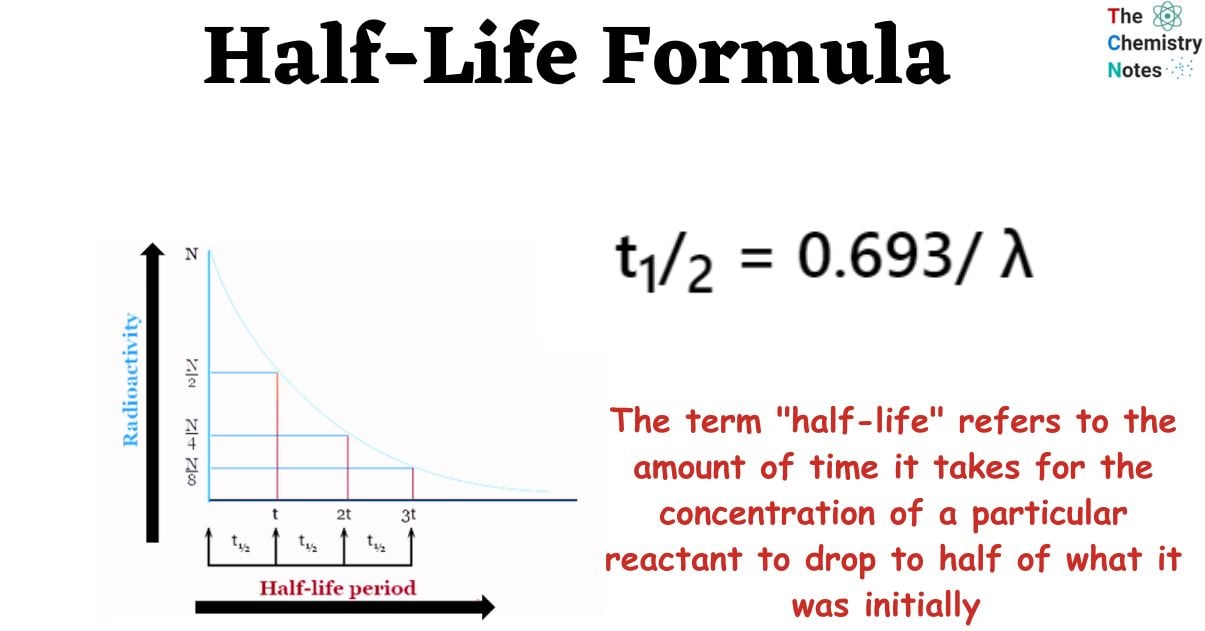
Half life Formula Derivation Application Examples
https://scienceinfo.com/wp-content/uploads/2023/06/Half-Life-Formula.jpg

Half Life Chemistry Formula
https://d20khd7ddkh5ls.cloudfront.net/activity_lead_into_half-lives.png

Half Life Equation Derivation YouTube
https://i.ytimg.com/vi/oSM6nyil7Q8/maxresdefault.jpg
The half life of the radioactive element Strontium 90 is 37 years In 1950 15 kilograms of this element released accidentally How do you determine the formula which shows the mass remaining after t years The half life is the length of time that it takes for half of an initial sample to undergo a change Usually this is the radio active decay of a specific atomic weight of an element For example the half life of Uranium 238 is 4 46 billion years The formula for half life which gives the number of remaining atoms after a time t and
[desc-10] [desc-11]
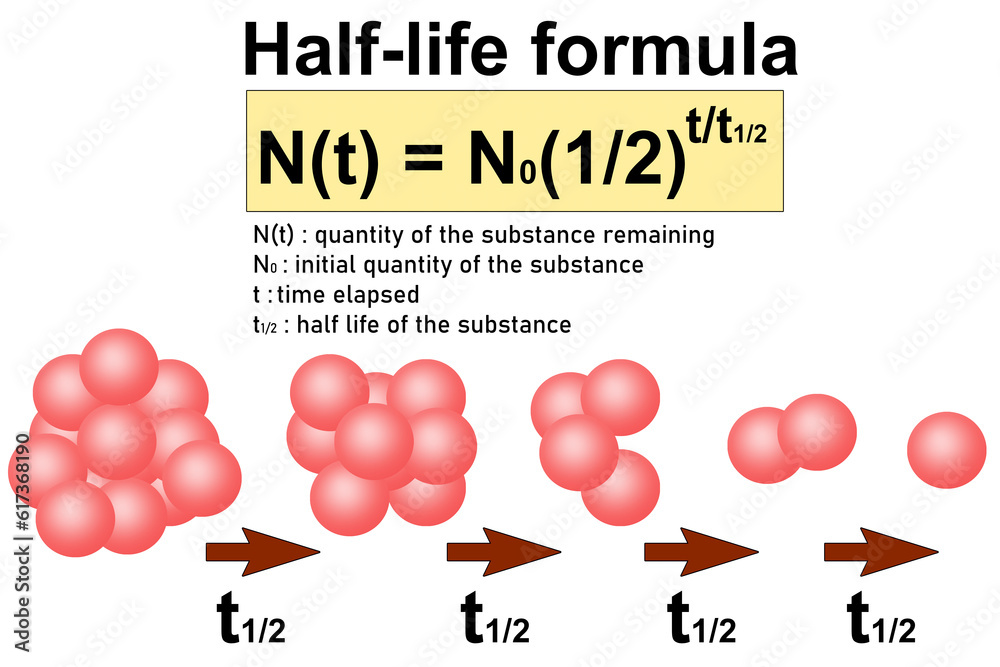
Half Life Formula And Radioactive Decay Diagram Stock Illustration
https://as2.ftcdn.net/v2/jpg/06/17/36/81/1000_F_617368190_rnR7OEA3EKbccX8raiMPEvzPloaD991s.jpg
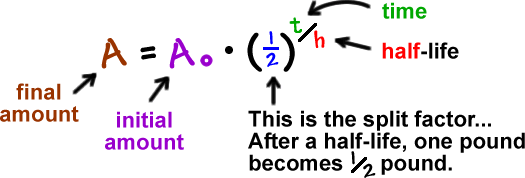
Half Life Formula
https://useruploads.socratic.org/bflWNTv9RPS7dTQHyjgA_13-exponentials-01.gif

https://socratic.org › questions › what-are-the-formulas-for-half-life-in-ex…
The formulas for half life are t ln2 and t tln2 ln N 0 N t The equation for exponential decay is 1 N t N 0e t where N 0 is the initial quantity N t is the quantity at time t is the exponential decay constant We can solve this for 2 1 tln N 0 N t And the formulas for half life t are 3 t ln2 and 4 t tln2 ln N 0 N t If you know the

https://socratic.org › questions
Half life is defined as the time after which half of a sample of a radioactive material will have decayed In other words if you start with 1 kg of material with a half life of 1 year then after 1 year you will have 500g After another year you will have half of that or 250 g After another year you will have 125 g and so on
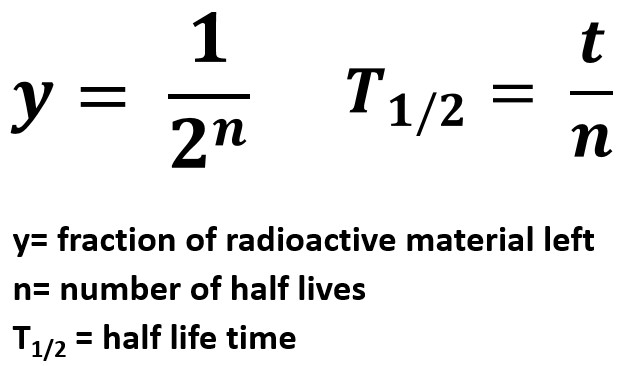
Half Life StickMan Physics

Half Life Formula And Radioactive Decay Diagram Stock Illustration
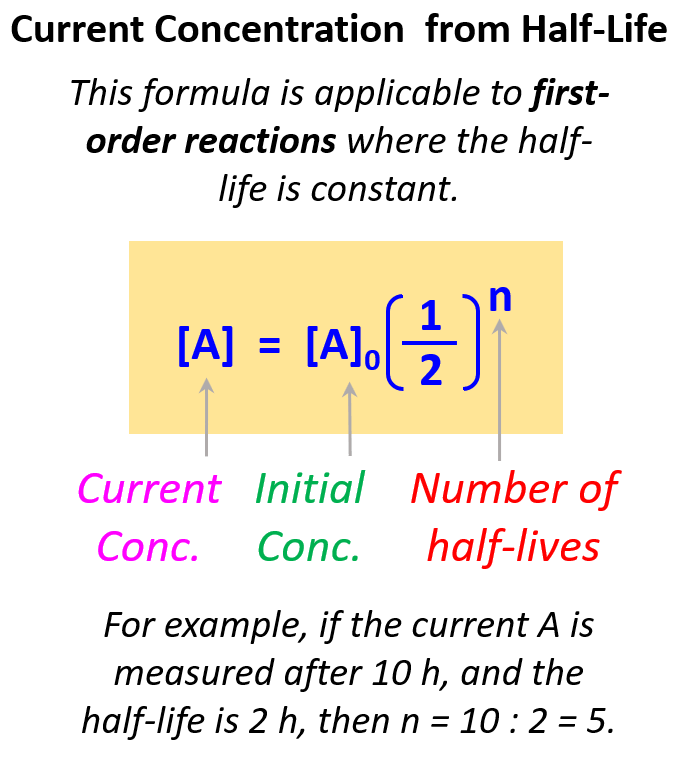
Half Life Formula

Half Life Formula Pharmacology Collen Gerald
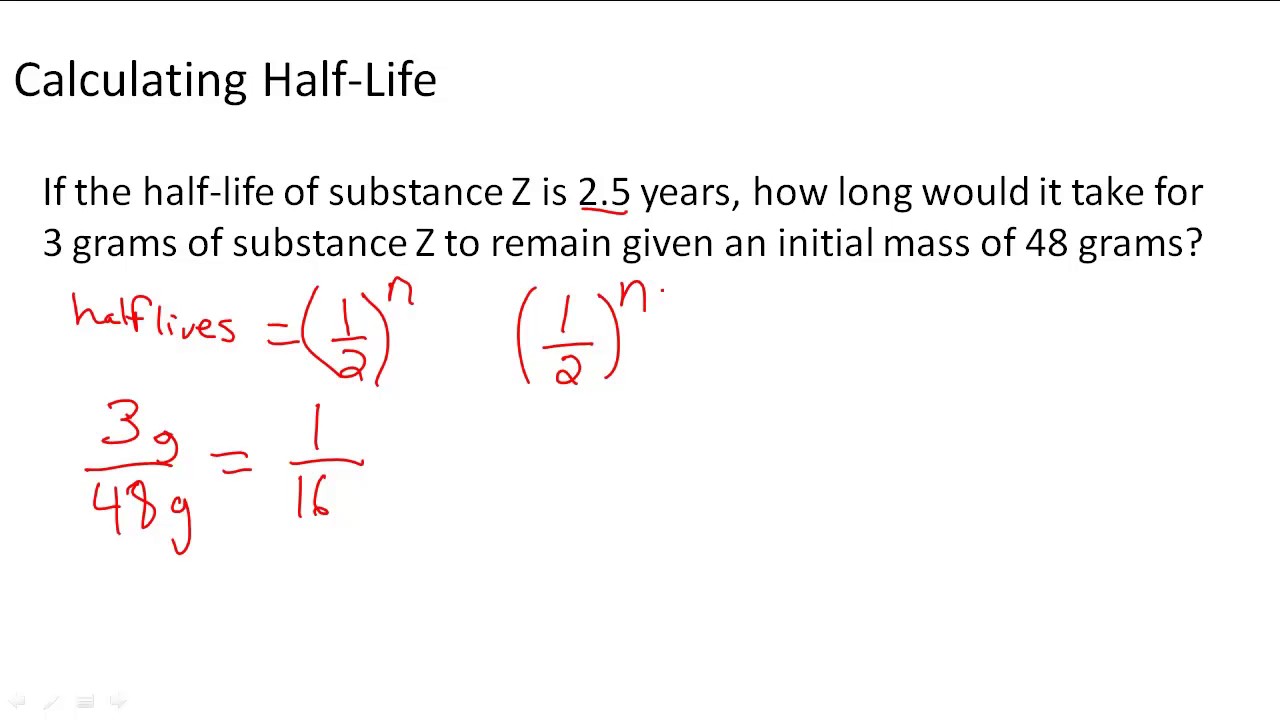
How To Find Half Life Radioactive Decay Haiper
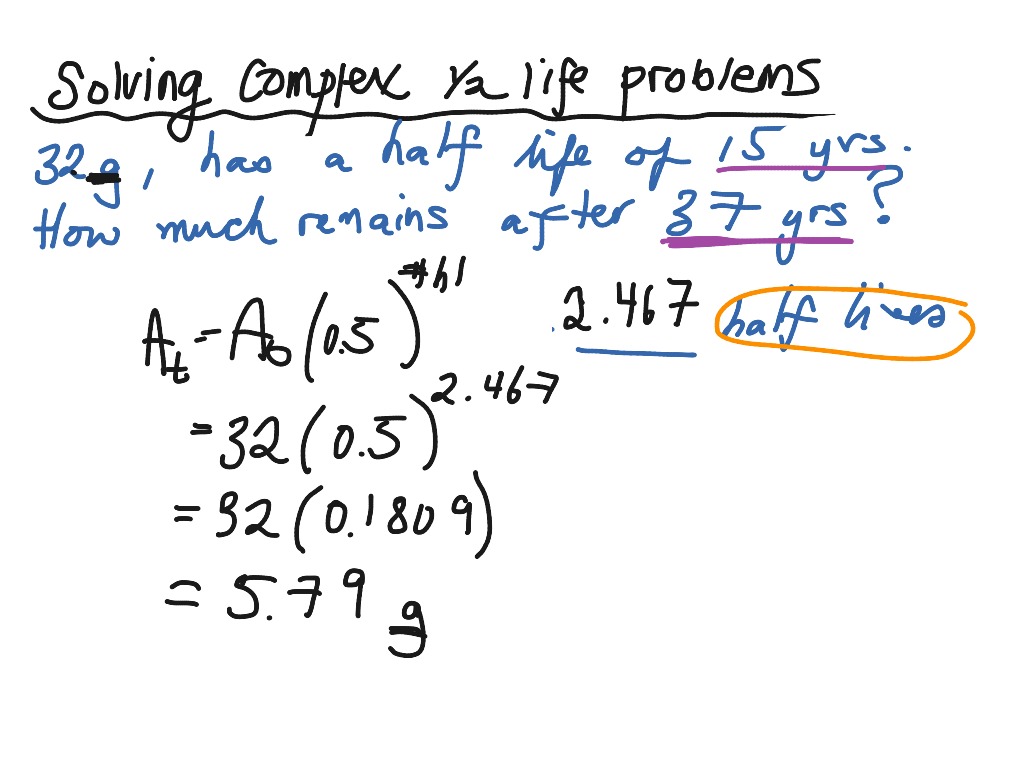
Using The Half life Formula For More Complex Problems Science

Using The Half life Formula For More Complex Problems Science

Half Life Formula Definition And Well derived Equation Chemistry Notes

Understanding Half life Formulas With Example YouTube
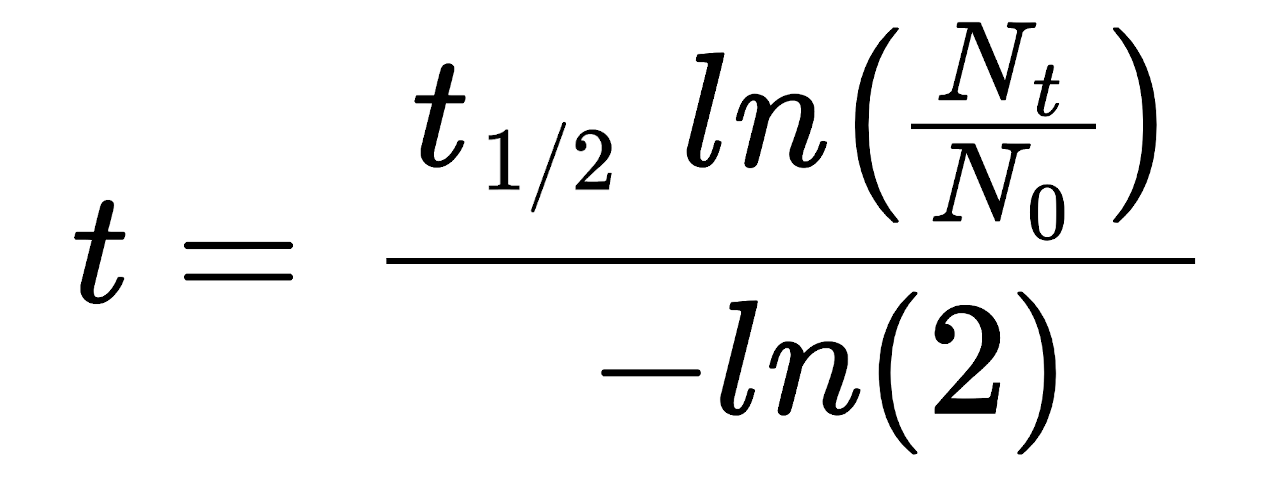
Half Life Calculator Inch Calculator
Half Life Formula - You could use this formula Where Th half life M the beginning amount M the ending amount One example of how to use the equation One of the Nuclides in spent nuclear fuel is U 234 an alpha emitter with a half life of 2 44 x10 5 years