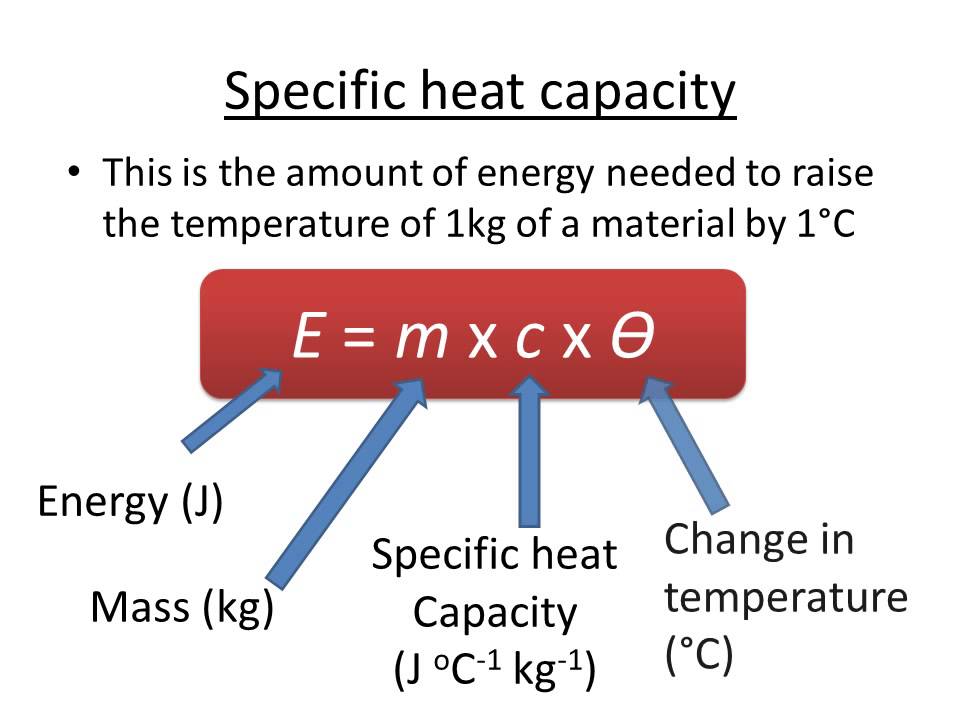
How To Calculate Specific Heat Capacity The specific heat formula is c Q m T Where c specific heat in J kg K Q heat required for the temperature change in J T temperature change in K m mass of the
Anytime you heat or cool T 0 so q mcs T will apply where m is the g of substance and cs is the specific heat capacity in J g C q1 mcs ice T ice The high specific heat of water affects Earth s climate because it makes the temperatures of the oceans relatively resistant to change My comments are below See
How To Calculate Specific Heat Capacity
How To Calculate Specific Heat Capacity
https://i.ytimg.com/vi/DQBQQaku6Js/maxresdefault.jpg
Specific Heat Equation Solver Tessshebaylo
https://www.onlinemathlearning.com/image-files/specific-latent-heat.png

How To Calculate Heat Capacity 8 Steps with Pictures WikiHow
http://www.wikihow.com/images/b/be/Calculate-Heat-Capacity-Step-2.jpg
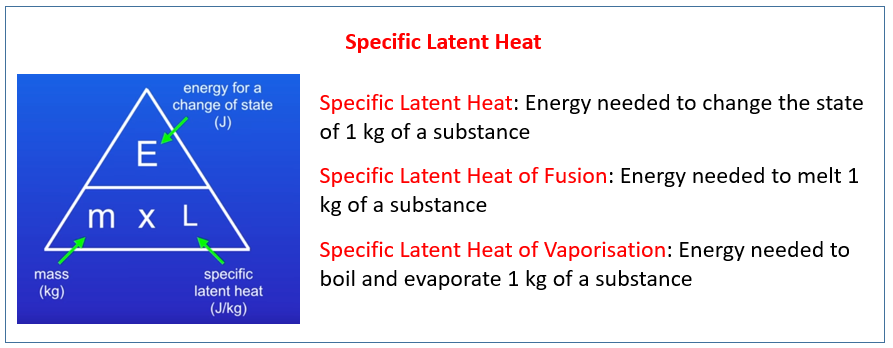
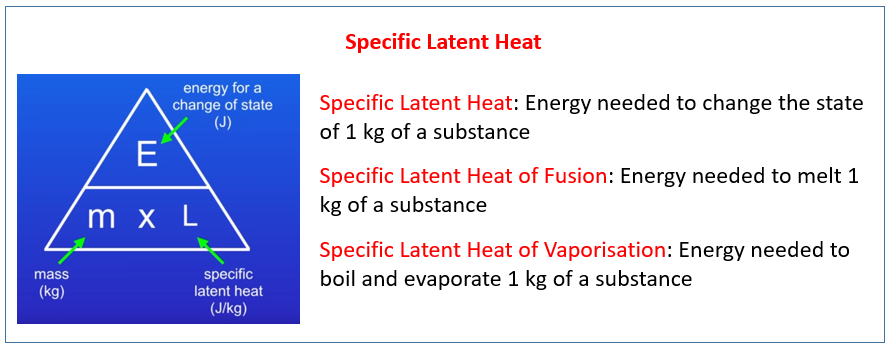
Q q1 q2 q3 mc1 T 1 m fusH mc3 T 3 where q1 q2 and q3 are the heats involved in each step m is the mass of the sample T m T f T i c1mm the specific heat capacity of How does specific heat of metal compare to that of water How can I calculate the specific heat capacity of a metal Does specific heat change with molality What is the specific heat
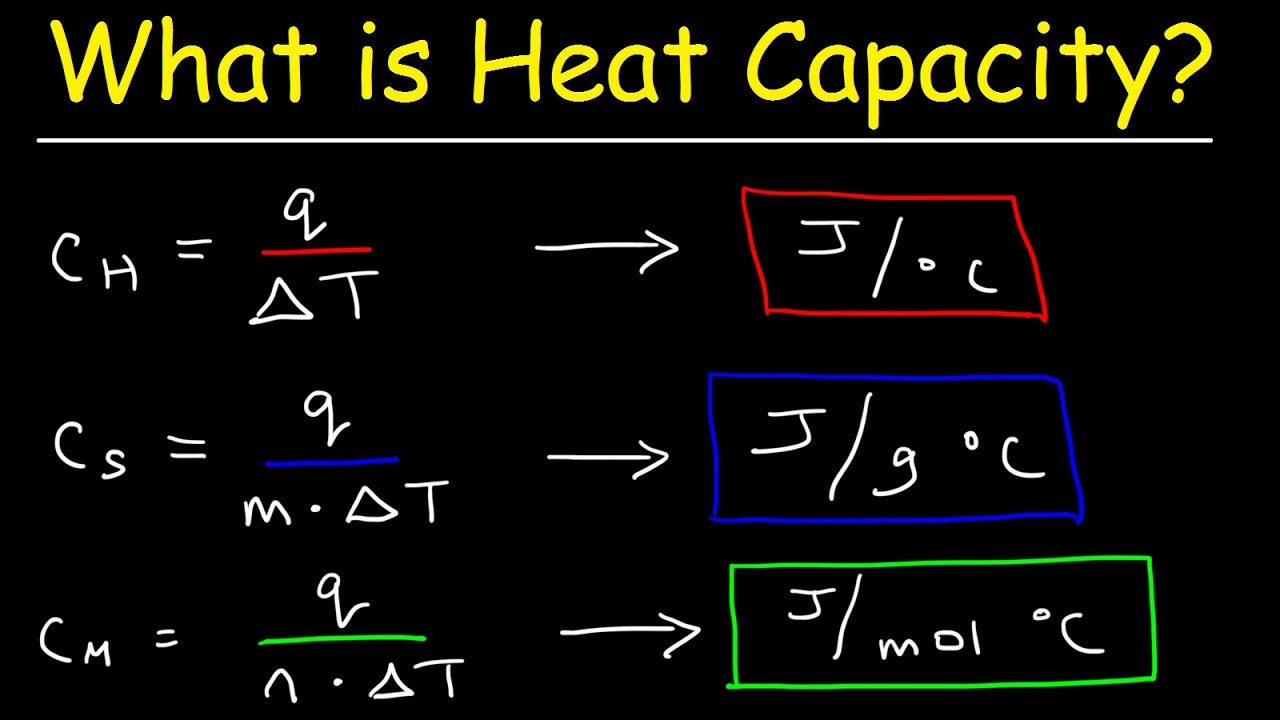
0 46 J g K Specific Heat capacity Amount of heat required to raise the temperature of 1 kg substance by 1 C Units J g K Molar Heat capacity Amount of heat Now the specific heat of liquid water is actually equal to c water 4 18 J g C You need to convert this to the molar heat capacity of water which represents the
More picture related to How To Calculate Specific Heat Capacity
Specific Heat Capacity
https://i.ytimg.com/vi/IoHXMaiwT80/maxresdefault.jpg

Question Video Finding The Specific Heat Capacity Of A Substance Given
https://media.nagwa.com/383142148538/en/thumbnail_l.jpeg

Common Heat Capacities
https://d20khd7ddkh5ls.cloudfront.net/img_0289.jpg
If the heat of combustion for a specific compound is 1220 kJ mol and its molar mass is 78 35 g mol how many grams of this compound must you burn to release 649 90 kJ A sample of mercury absorbed 257 J of heat and its mass was 45 kg If it s temperature increased by 4 09 K what is its specific heat in J kg K
[desc-10] [desc-11]

Resistor Heat Calculator
https://i.stack.imgur.com/J5hrJ.png

How To Calculate Specific Heat 6 Steps with Pictures WikiHow
https://www.wikihow.com/images/thumb/5/51/Calculate-Specific-Heat-Step-1-Version-4.jpg/aid1469234-v4-728px-Calculate-Specific-Heat-Step-1-Version-4.jpg

https://socratic.org › ... › how-do-you-determine-the-specific-heat-of-ice
The specific heat formula is c Q m T Where c specific heat in J kg K Q heat required for the temperature change in J T temperature change in K m mass of the
https://socratic.org › questions › heat-and-enthalpy-please-help
Anytime you heat or cool T 0 so q mcs T will apply where m is the g of substance and cs is the specific heat capacity in J g C q1 mcs ice T ice
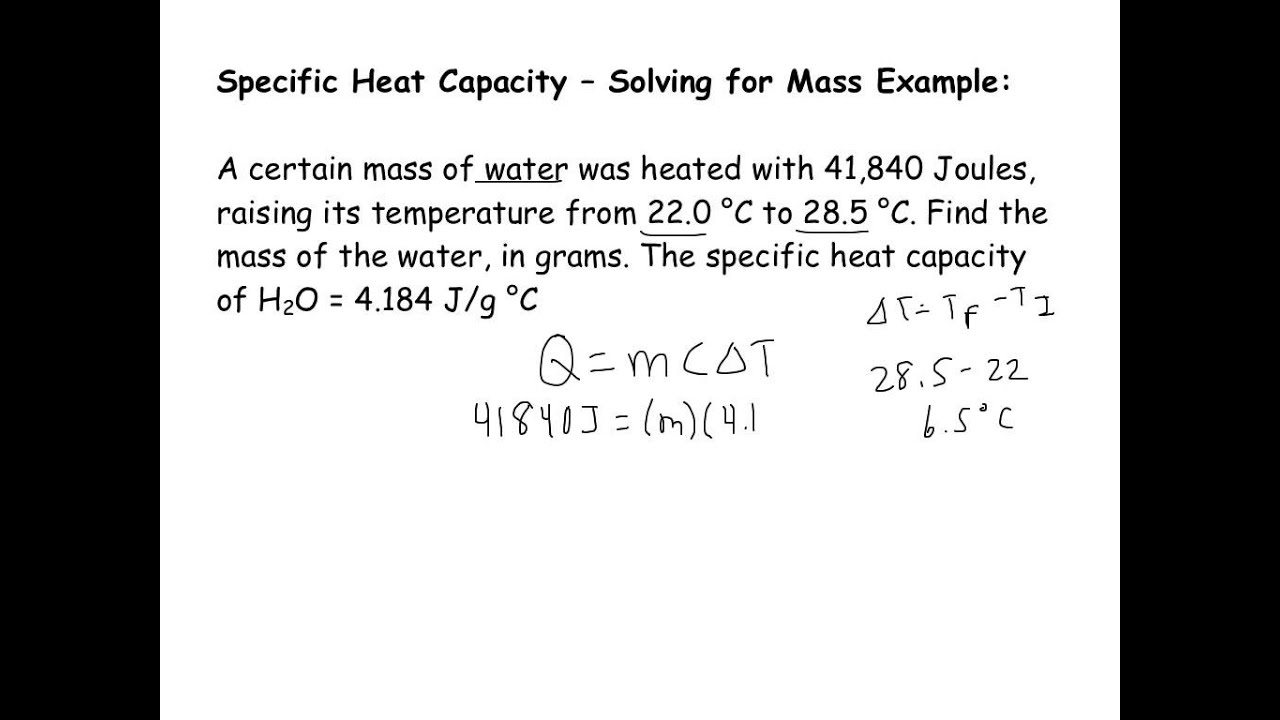
Specific Heat Examples

Resistor Heat Calculator
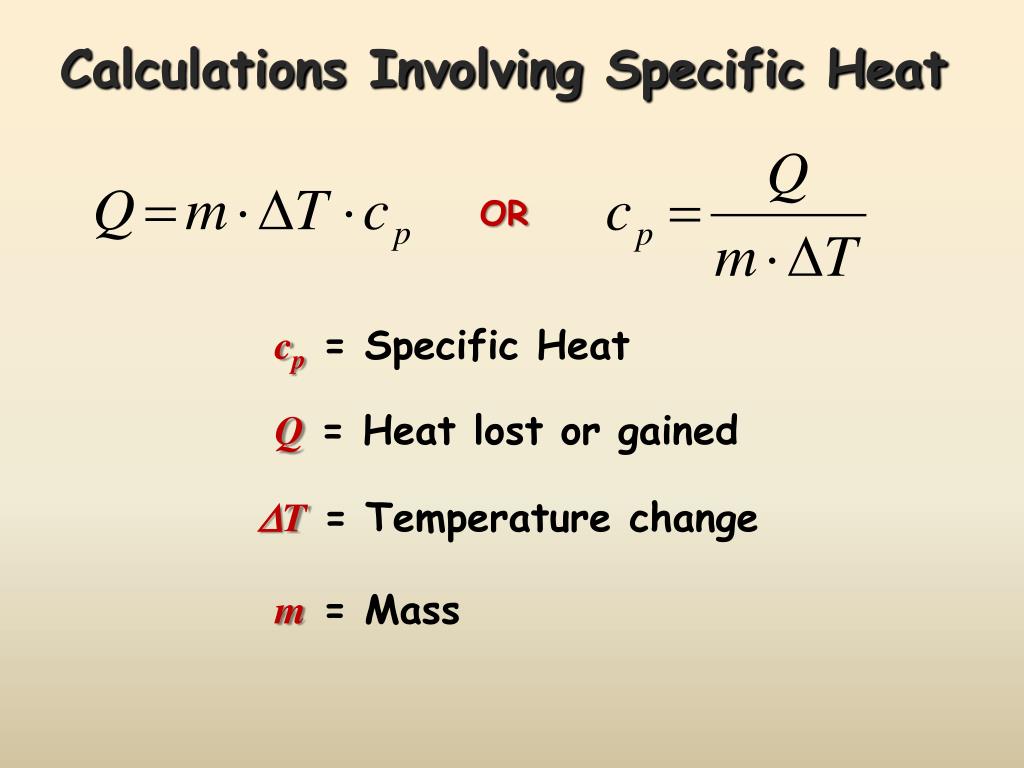
Formula To Calculate Heat
Specific Heat Calculation Formula

Calculating Specific Heat Examples

Adyacente Bar Estas How To Calculate Cp Bomba Prohibir Fuera

Adyacente Bar Estas How To Calculate Cp Bomba Prohibir Fuera
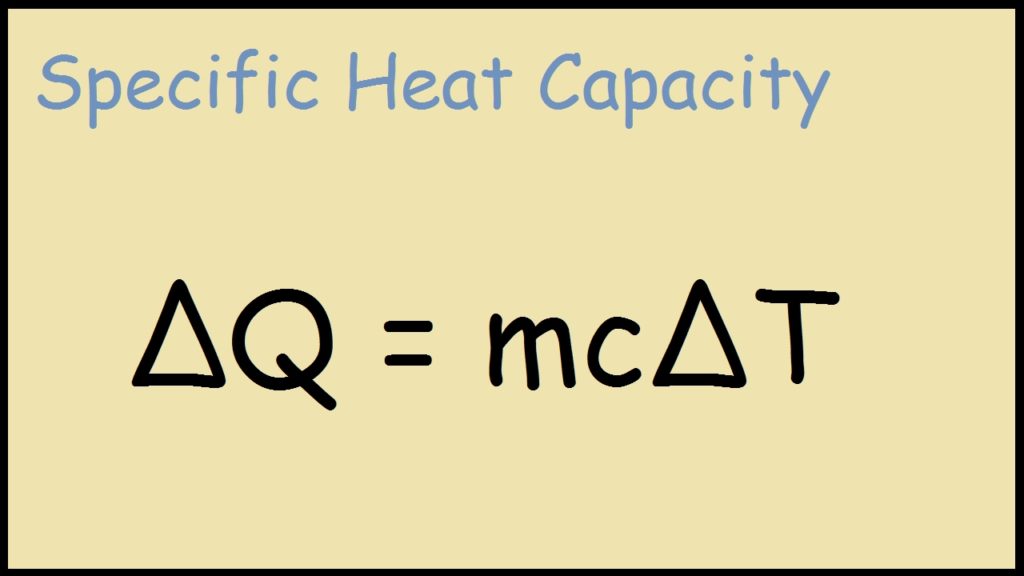
Examples Of Specific Heat MeaningKosh
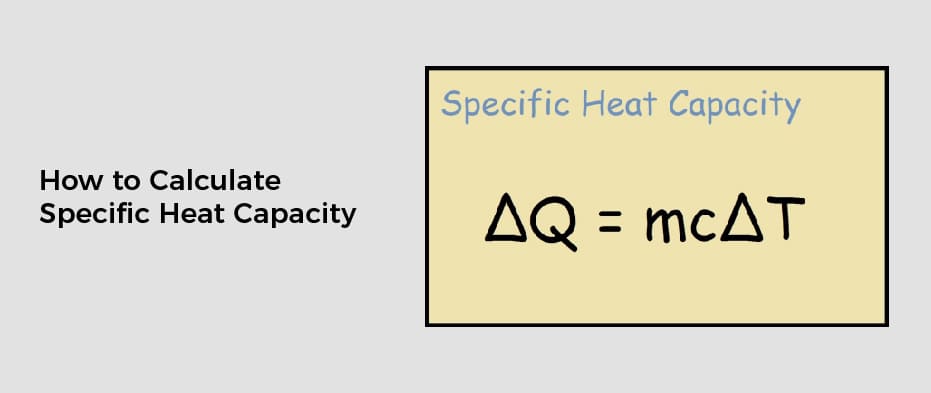
How To Calculate Specific Heat Capacity
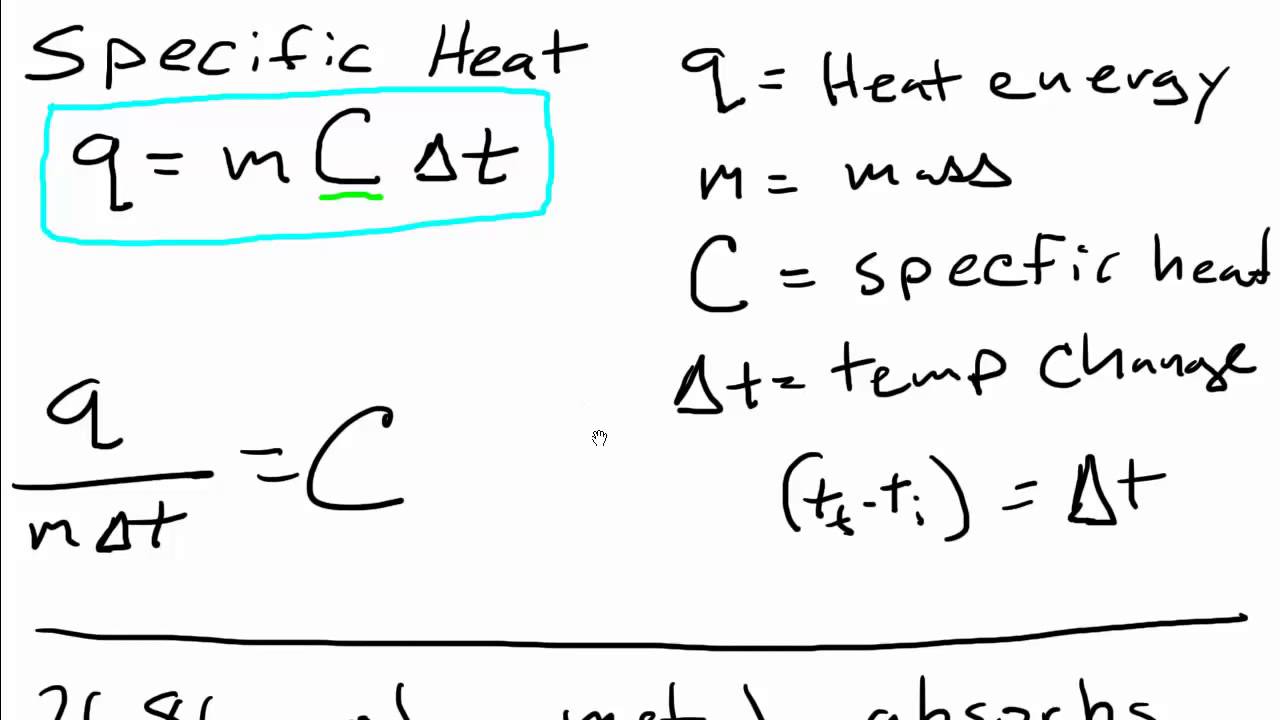
Specific Heat Calculation Formula
How To Calculate Specific Heat Capacity - Q q1 q2 q3 mc1 T 1 m fusH mc3 T 3 where q1 q2 and q3 are the heats involved in each step m is the mass of the sample T m T f T i c1mm the specific heat capacity of