Modified Rodnan Skin Score Sheet This score consists of an evaluation of patient s skin thickness rated by clinical palpation using a 0 3 scale 0 normal skin 1 mild thickness 2 moderate thickness 3 severe thickness with inability to pinch the skin
The modified Rodnan skin score mRSS is a measure of skin thickness and is used as a primary or secondary outcome measure in clinical trials of systemic sclerosis SSc scleroderma This article gives a brief history of the development of the mRSS outlines practical aspects of assessing mRSS in clinical trials and provides recommendations The modified Rodnan skin score mRSS is a measure of skin thickness and is used as a primary or secondary outcome measure in clinical trials of systemic sclerosis SSc This article gives a brief history of mRSS development outlines practical aspects of assessing mRSS in clinical trials and provides recommendations for Phase 2 and 3
Modified Rodnan Skin Score Sheet
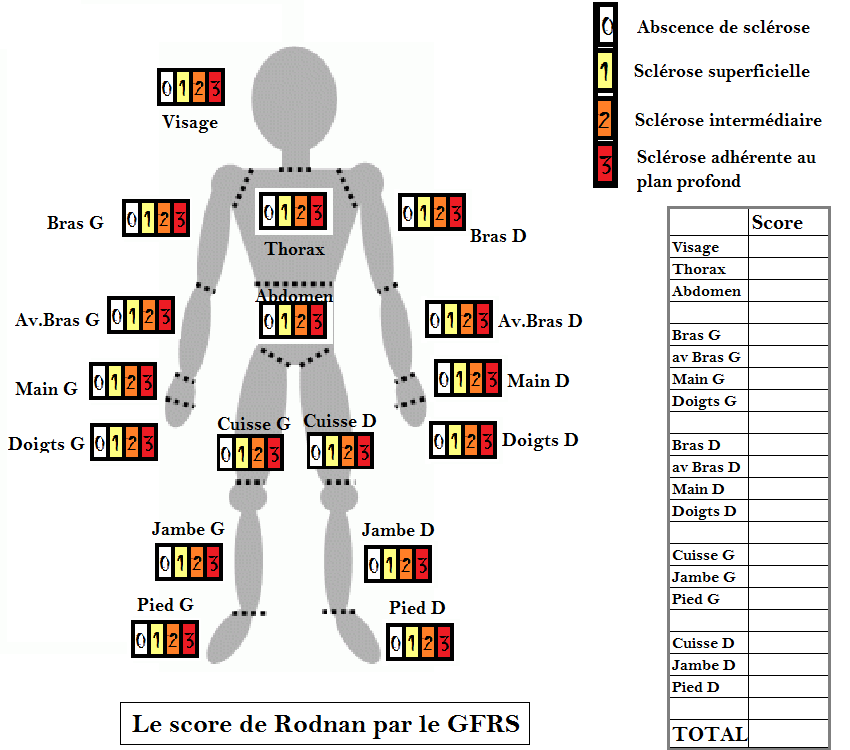
Modified Rodnan Skin Score Sheet
http://sclerodermie.net/wp-content/uploads/2014/07/score-Rodnan-GFRS-Pic.png
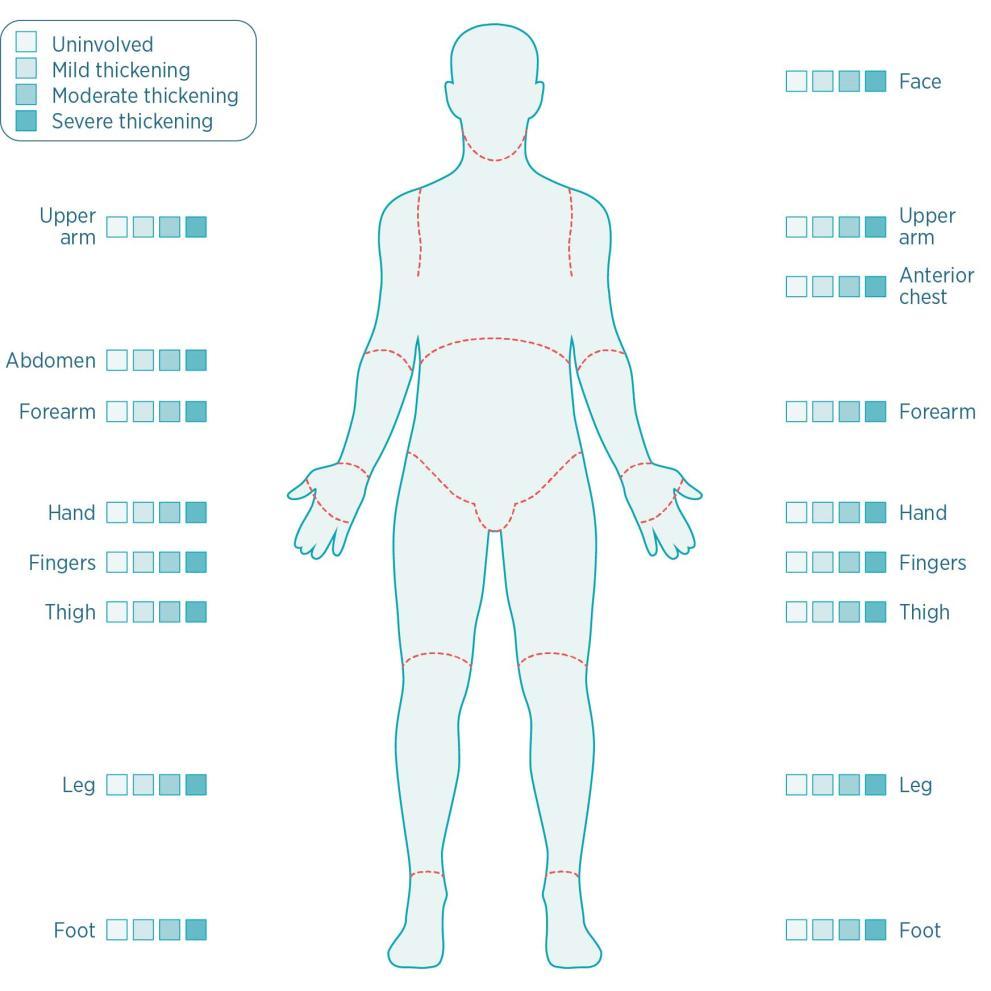
Skin Thickening Test More Than Scleroderma
https://patient.boehringer-ingelheim.com/more-than-scleroderma/sites/default/files/styles/bi_gds_medium/public/2022-06/2-3-5-modified_rodnan_skin_thickness_score_diagram-01_0.jpg?itok=atVZ7VD9
Modified Rodnan Skin Score Ann Rheum Dis 2007 Czirj k 966 9
https://imgv2-2-f.scribdassets.com/img/document/364201789/original/b888f84d22/1568876599?v=1
This state of art review provides a historical perspective of the development of the modified Rodnan skin score summarizes the performance of mRSS as an outcome measure provides guidance on assessingmRSS and makes recommendations for incorporation of the mR SS into clinical trials Modified Rodnan skin score mRSS is assessed in 17 different areas Case report form to capture mRSS Feasible Easy to perform with no equipment and requires little time Face Validity Captures and measures skin thickness Content Validity Covers the affected body areas in SSc Construct validity Correlates with other measures of SSc such as durometer and ultrasound
The modified Rodnan skin score mRSS is a measure of skin thickness and is used as a primary or secondary outcome measure in clinical trials of systemic sclerosis scleroderma This state of art review provides a historical perspective of the development of the mRSS summarizes the performance of The modified Rodnan skin score mRSS is a measure of skin thickness and is used as a primary or secondary outcome measure in clinical trials of systemic sclerosis scleroderma This state of art review provides a historical perspective of the development of the mRSS summarizes the performance of mRSS as an outcome measure
More picture related to Modified Rodnan Skin Score Sheet
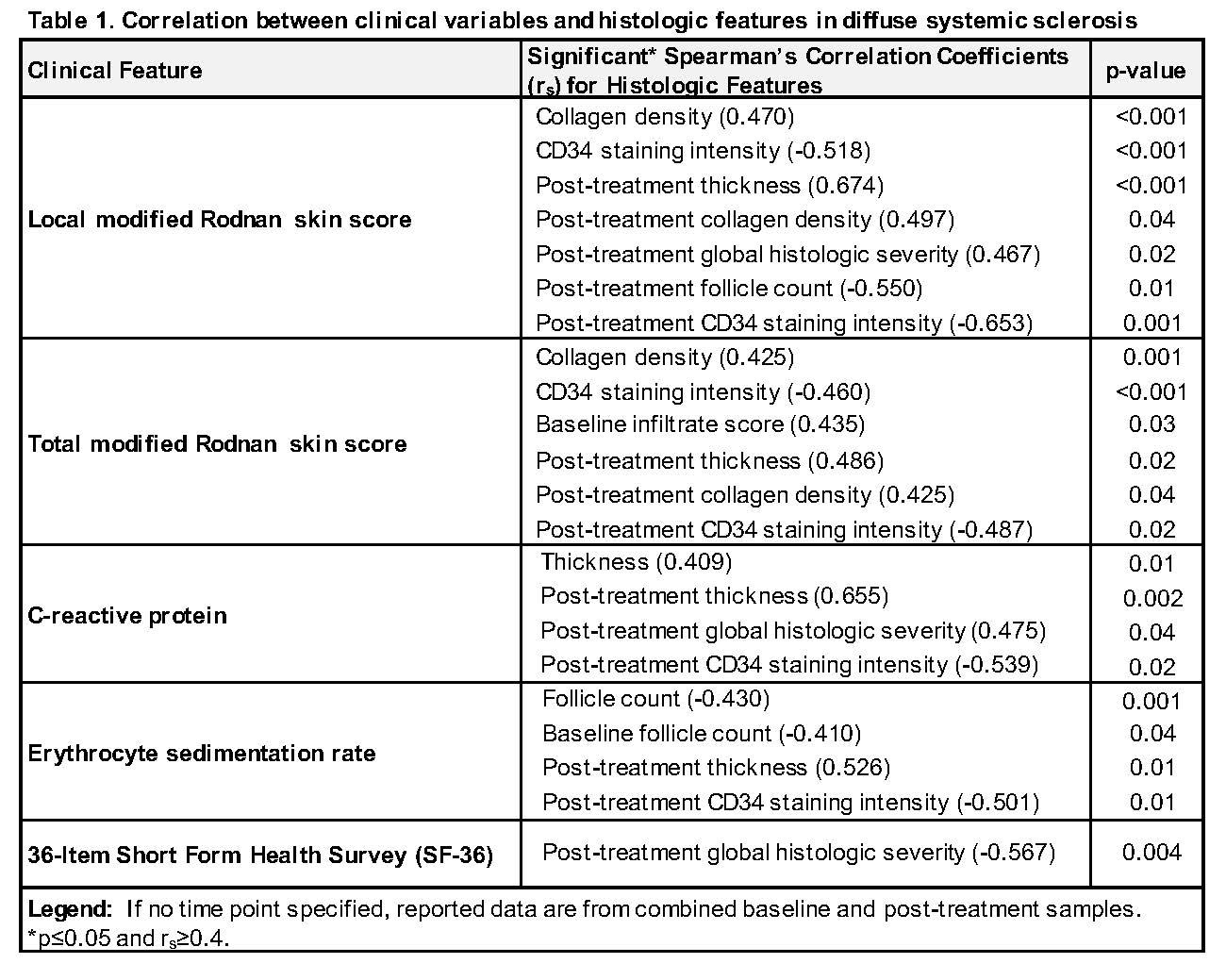
Histologic Features Correlate With The Modified Rodnan Skin Score
https://acrabstracts.org/wp-content/uploads/2019/08/GTUOHVCE-675971-1-ANY-3-.jpg

Correlation Between Modified Rodnan Skin Score Score And Young s
https://www.researchgate.net/profile/Durga_Misra/publication/318556915/figure/download/tbl1/AS:669353972342784@1536597831164/Correlation-between-modified-Rodnan-skin-score-score-and-Youngs-modulus-of-shear-wave.png

Scleroderma Clinical Trials Consortium SCTC Rodnan Skin Score Training
https://sclerodermaclinicaltrialsconsortium.org/images/2018/12/11/shutterstock_334458899.jpg
Modified Rodnan skin scores mRSS of 8 systemic sclerosis patients The mRSS is calculated by summation of measurements of skin thickness in 17 different body sites including the face upper arms forearms dorsum of hands fingers chest abdomen thighs legs and feet The modified Rodnan skin score mRSS a measure of skin thickness is the primary outcome measure in the majority of clinical trials of diffuse cutaneous SSc dcSSc Measurement of skin thickness is used as surrogate measure of disease severity and mortality in patients with dcSSc
To assess the course of modified Rodnan skin score MRSS in patients with diffuse cutaneous systemic sclerosis dcSSc with different baseline disease durations defined from the date of onset of first non Raynaud s phenomenon symptom in 3 large randomized controlled trials RCT At present the most important validated method gold standard for measuring the dermal skin thickness is the modified Rodnan skin score mRSS 5 Skin histology also seems to be an appropriate method for the evaluation of skin thickness in scleroderma

Scleroderma Clinical Trials Consortium SCTC Rodnan Skin Score Training
https://sclerodermaclinicaltrialsconsortium.org/images/2018/12/11/calcinosis_xray2.jpg

Slide 14
https://www.sec.gov/Archives/edgar/data/1595097/000119312516767051/g288604ex99_2s14g1.jpg
https://rheumguide.ca/uploads/1/2/7/1/127151112/mrss-pa…
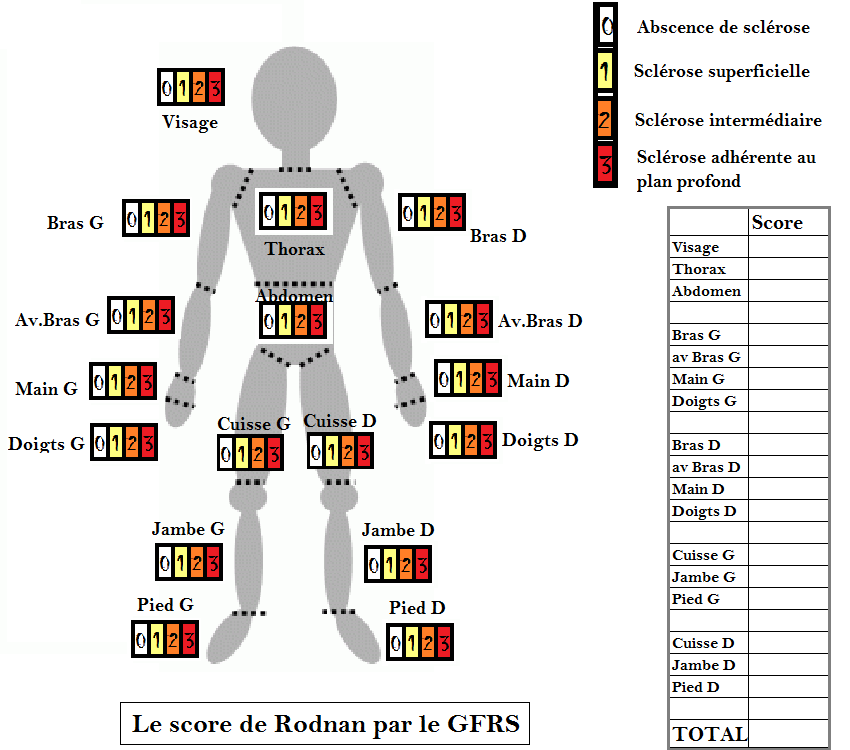
This score consists of an evaluation of patient s skin thickness rated by clinical palpation using a 0 3 scale 0 normal skin 1 mild thickness 2 moderate thickness 3 severe thickness with inability to pinch the skin

https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/28516167
The modified Rodnan skin score mRSS is a measure of skin thickness and is used as a primary or secondary outcome measure in clinical trials of systemic sclerosis SSc scleroderma This article gives a brief history of the development of the mRSS outlines practical aspects of assessing mRSS in clinical trials and provides recommendations
Rheumatoid Arthritis And Modified Rodnan Skin Score Encounter Forms For

Scleroderma Clinical Trials Consortium SCTC Rodnan Skin Score Training

PDF Skin Model For Improving The Reliability Of The Modified Rodnan

PDF Minimal Clinically Important Differences For The Modified Rodnan

A The Modified Rodnan Skin Score mRSS Was Higher In Skin Biopsies

What Is Modified Rodnan Skin Scoring In scleroderma Scoring Of 1 7

What Is Modified Rodnan Skin Scoring In scleroderma Scoring Of 1 7

Flow chart RP Raynaud s Phenomenon MRSS Modified Rodnan Skin Score

Modified Rodnan Skin Score Online Calculator For Use In Systemic

A Absolute Change In Modified Rodnan Skin Score mRSS From Baseline
Modified Rodnan Skin Score Sheet - The modified Rodnan skin score mRSS is a measure of skin thickness and is used as a primary or secondary outcome measure in clinical trials of systemic sclerosis scleroderma This state of art review provides a historical perspective of the development of the mRSS summarizes the performance of mRSS as an outcome measure
