Neutralization Reaction Simple Definition Science The reaction between an acid and a base is termed as neutralization reaction This is because when an acid reacts with a base they cancel out or nullify each other In general it can be
0 16 g of dibasic acid required 25 ml of decinormal N aOH solution for complete neutralisation The molecular weight of the acid will be 32 64 128 256 Enthalpy change of neutralization is always measured per mole of water formed Enthalpy changes of neutralization are always negative as heat is released when an acid and alkali
Neutralization Reaction Simple Definition Science
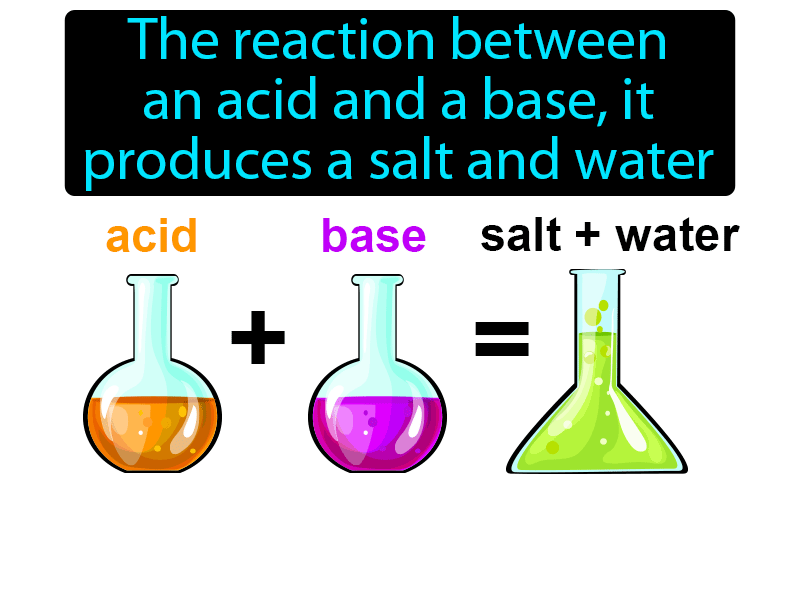
Neutralization Reaction Simple Definition Science
https://gamesmartz.com/upload/subjects/science/800-no-text/neutralization.png
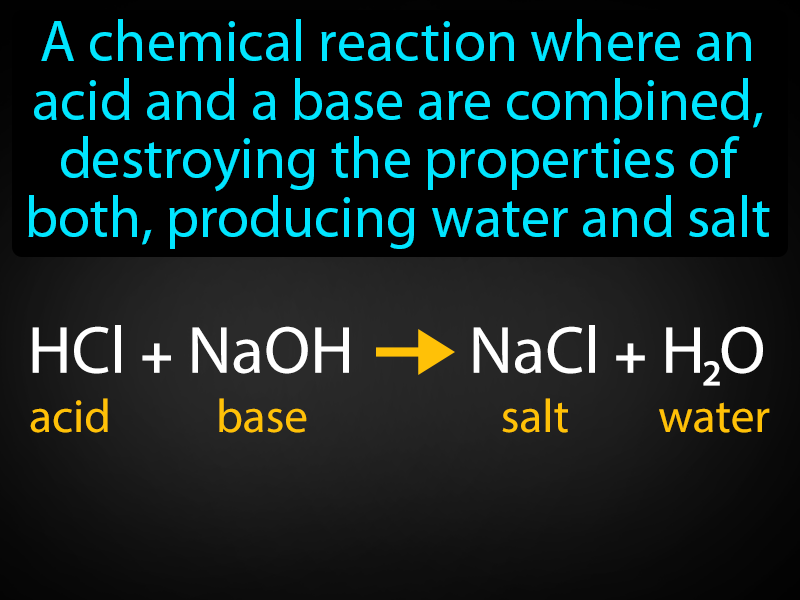
Neutralization Reaction Definition Image GameSmartz
https://gamesmartz.com/upload/subjects/science/800-no-text/neutralization-reaction.png

Neutralization Reaction Definition Examples Applications 45 OFF
https://media.geeksforgeeks.org/wp-content/uploads/20230301135903/Neutralization-Reaction-1.jpg
The neutralization of strong acid and strong bases simply involves the combination of H ion from acid and O H ions from the base to form water molecules it has been found Assertion Heat of neutralisation of H N O3 and N aOH is same as that of H Cl and KOH Reason Both H N O3 and H Cl are strong acids and N aOH and KOH are strong bases Both Assertion
The reaction in which an acid and a base react to give a salt and water is called neutralization reaction Neutralization reactions are exothermic in nature The heat change when one gram Neutralisation of a carbonate with an acid produces carbon dioxide gas but not with an oxide or hydroxide
More picture related to Neutralization Reaction Simple Definition Science

Neutralization Reaction Worksheet Online Exercise For Live Worksheets
https://www.liveworksheets.com/sites/default/files/styles/worksheet/public/def_files/2021/4/6/104060149041293107/104060149041293107001.jpg?itok=-WKI8HDv

Haloform Reaction NROChemistry
https://nrochemistry.com/wp-content/uploads/2022/09/Haloform-Reaction1-1280x1280.jpeg

Free Printable Neutralization Reactions Worksheets Worksheets Library
https://worksheets.clipart-library.com/images2/neutralization-reaction-worksheet/neutralization-reaction-worksheet-11.png
Adding an acid to water decreases the extent to which water dissociates and leads to decrease in the concentration of hydroxide ions and increase in concentration of hydronium ions 2H 2O l The ammonia evolved from the treatment of 0 30 g of an organic compound for the estimation of nitrogen was passed in 100 mL of 0 1 M sulphuric acid The excess of acid required 20 mL of
[desc-10] [desc-11]
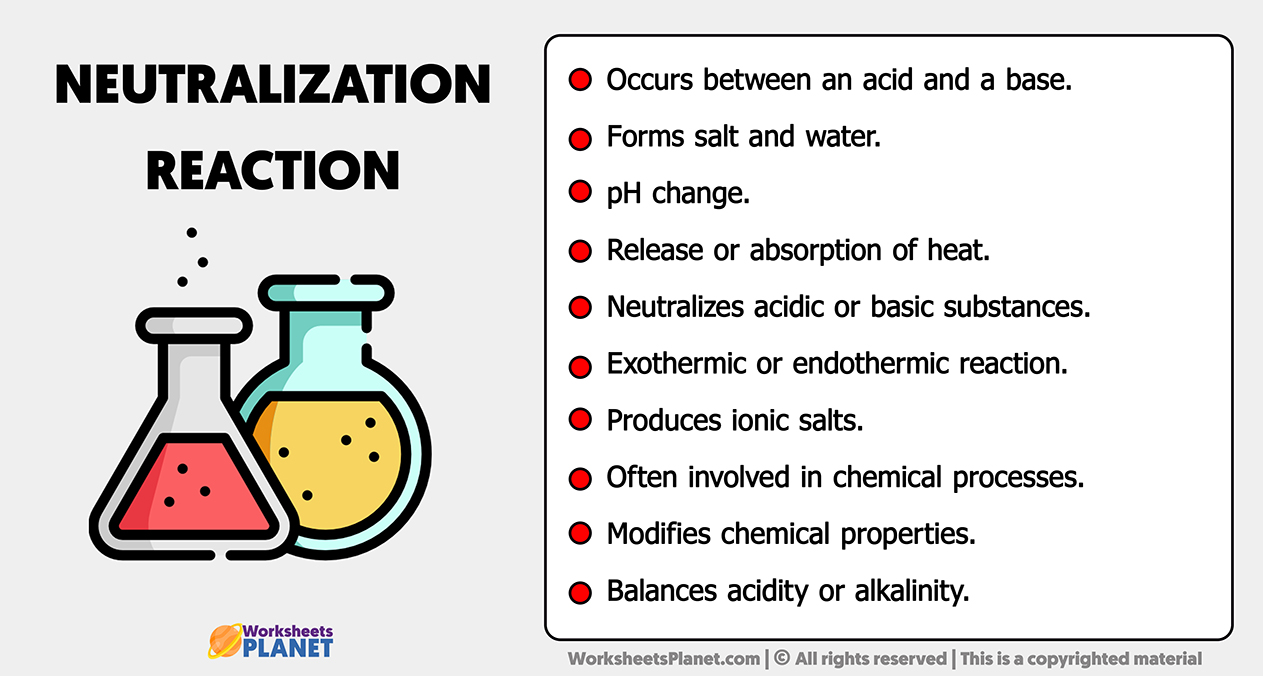
Neutralization Reaction Characteristics
https://www.worksheetsplanet.com/wp-content/uploads/2023/11/Neutralization-Reaction-Characteristics.jpg

Neutralisation Meaning
https://i0.wp.com/www.abcworksheet.com/wp-content/uploads/2023/08/what-is-neutralization-reaction.jpg?resize=1024%2C725&ssl=1

https://www.toppr.com › ask › question › what-is-meant-by-neutralizatio…
The reaction between an acid and a base is termed as neutralization reaction This is because when an acid reacts with a base they cancel out or nullify each other In general it can be

https://www.toppr.com › ask › question
0 16 g of dibasic acid required 25 ml of decinormal N aOH solution for complete neutralisation The molecular weight of the acid will be 32 64 128 256

What Are Precipitates Definition Overview Expii

Neutralization Reaction Characteristics

Neutralization

Neutralization
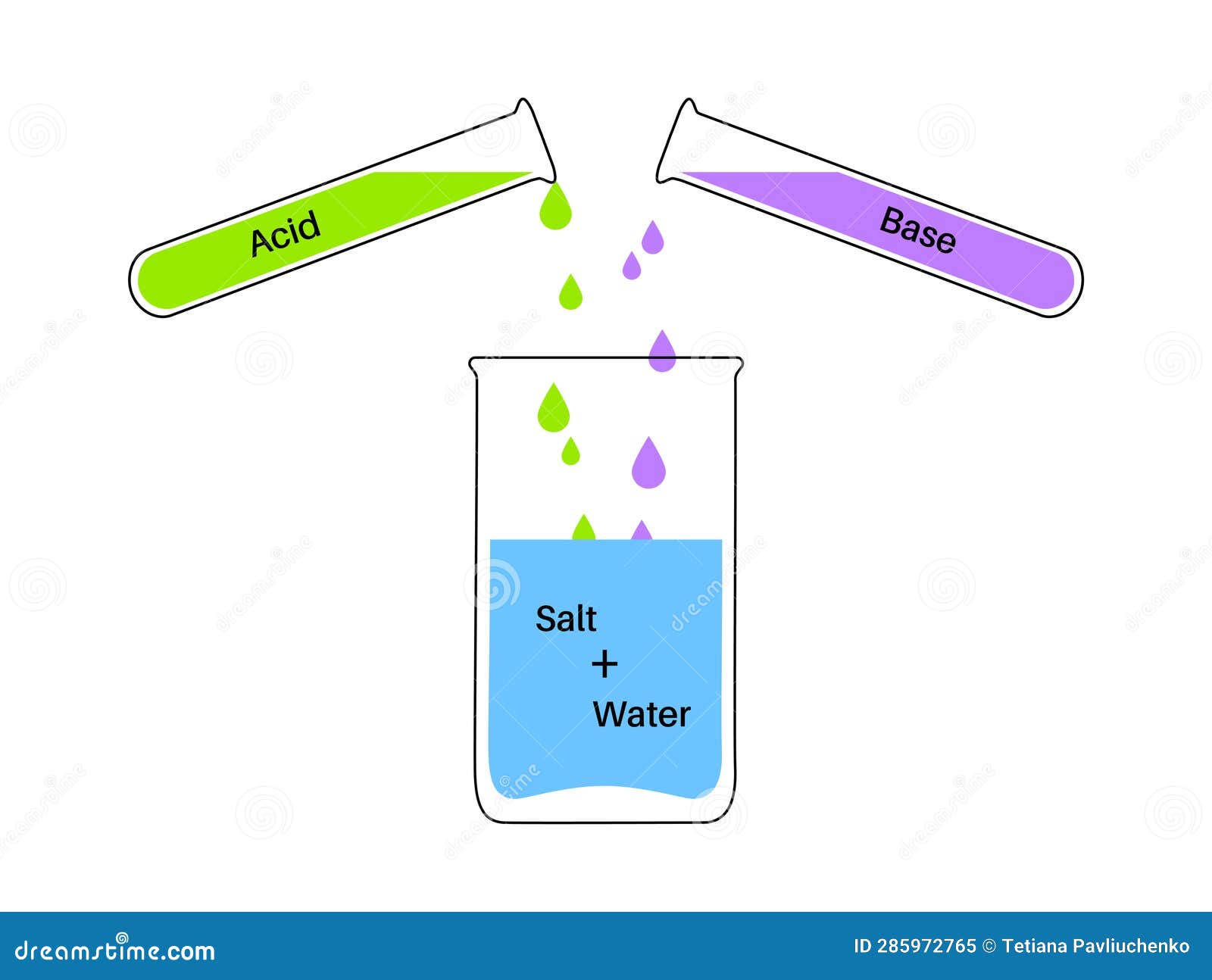
Reaction Neutralization Poster Vector Illustration CartoonDealer

How To Write Chemical Equations For Neutralization Reactions Tessshebaylo

How To Write Chemical Equations For Neutralization Reactions Tessshebaylo

What Are Endothermic Reactions with Examples Video

Neutralization Reaction
Neutralization Reaction Definition Equation Examples Applications
Neutralization Reaction Simple Definition Science - [desc-14]