P Vs 1 V Graph P vs 1 V 3 P V vs P Interpret each graph in terms of a law View Solution Q2 Graph of V vs I is given
P v s dfrac 1 V graph is straight line for adiabatic process A Both Assertion and Reason are correct and Reason is the correct explanation for Assertion P v s dfrac 1 V graph is straight line for adiabatic process View Solution Q3 Reason The
P Vs 1 V Graph

P Vs 1 V Graph
https://edurev.gumlet.io/ApplicationImages/Temp/8687130_f59720b6-c442-46b6-a0b0-fdc32d68c607_lg.png?w=400&dpr=2.6
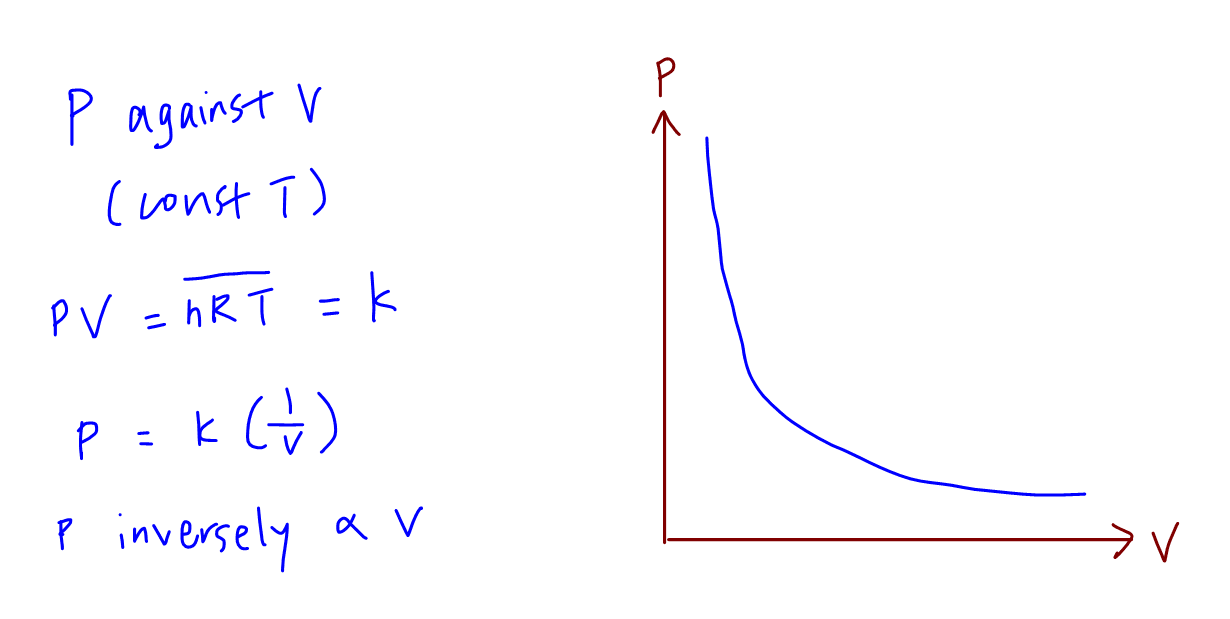
Avogadros Law Graph
https://chemistryguru.com.sg/images/ideal_gas_graph_sketching-P_against_V_at_constant_T_graph.png
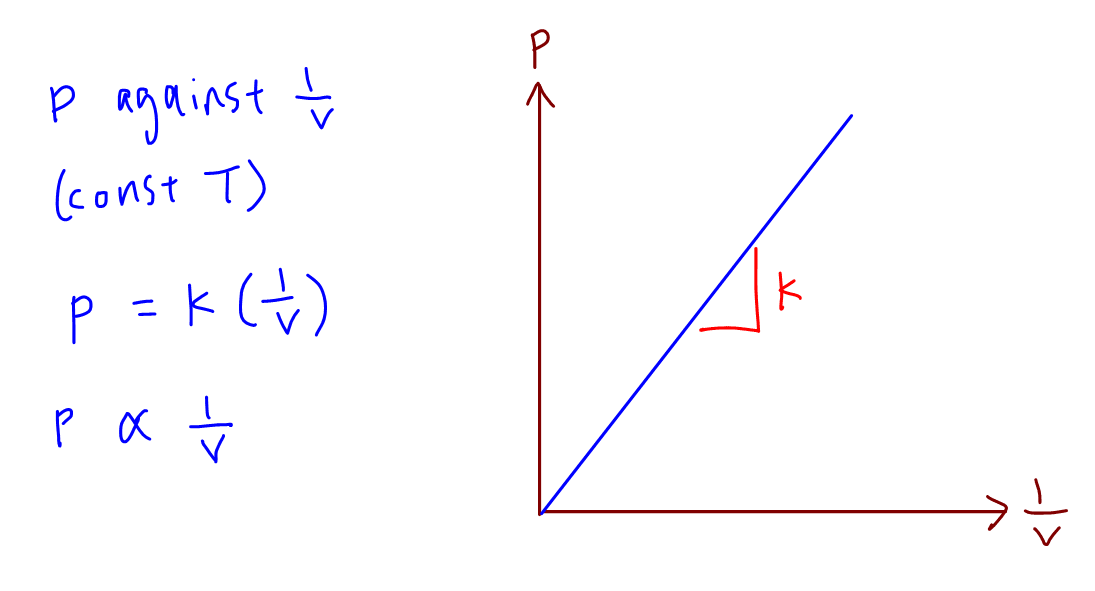
Ideal Gas Graph Sketching
https://chemistryguru.com.sg/images/ideal_gas_graph_sketching-P_against_V-1_at_constant_T_graph.png
A The P V graph at constant temperature is a rectangular hyperbola b The P V graph is a straight line parallel to the Y axis c P V graph at constant temperature is a straight line passing through the origin d V T graph at constant pressure is a straight line passing through the origin P is the pressure V is the volume n is the number of moles of amount of substance of gas R is the ideal or universal gas constant T is the temperature of the gas Since temperature is kept constant the RHS of the equation is a constant PV Constant As such the graph of PV against P should be a straight line parallel to the P axis
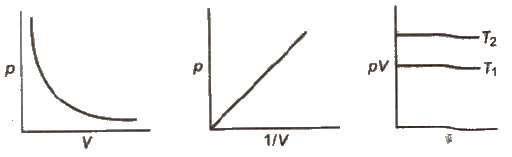
For the values of P vs V plotted at three different temperatures the following three curves were obtained Arrange the values of the temperatures T 1 T 2 and T 3 in the correct order View Solution The graph of P vs 1 V for a finite amount of gas at a constant temperature is a View Solution Q5
More picture related to P Vs 1 V Graph
Class 11 Chemistry Chapter States Of Matter Notes STRIKE NTSE OFFICIAL
https://farm4.staticflickr.com/3914/14985103531_c1e6856f1a_o.jpg
Solved Question Part Submissions Used A Very Useful Way Of Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/60f/60fb1839-f8a2-420b-abc9-0f15d4b39cdf/phpYWTwrJ.png

Boyle s Law Definition Equation Examples And FAQs
https://media.geeksforgeeks.org/wp-content/uploads/20230427103101/Boyles-Law-2.png
The following graph correctly reflects log P vs log 1 V for a finite amount of gas at a constant According to Boyles law P V is constant Thus the graph is a straight line passing through the origin at a constant temperature as P is inversely proportional to V P V K
[desc-10] [desc-11]
Plots Of P Versus 1 V At Different Temperatures Toppr
https://haygot.s3.amazonaws.com/questions/1097642_93d052a0163d43208d8cda5be0c8432c.JPG
V And P Graph Of 1
https://d10lpgp6xz60nq.cloudfront.net/web-thumb/643183537_web.png

https://www.toppr.com › ask › question
P vs 1 V 3 P V vs P Interpret each graph in terms of a law View Solution Q2 Graph of V vs I is given

https://www.toppr.com › ask › question
P v s dfrac 1 V graph is straight line for adiabatic process A Both Assertion and Reason are correct and Reason is the correct explanation for Assertion

Draw A Graph Of Log P And Log 1 V For A Fixed Amount Of Gas At Cons

Plots Of P Versus 1 V At Different Temperatures Toppr
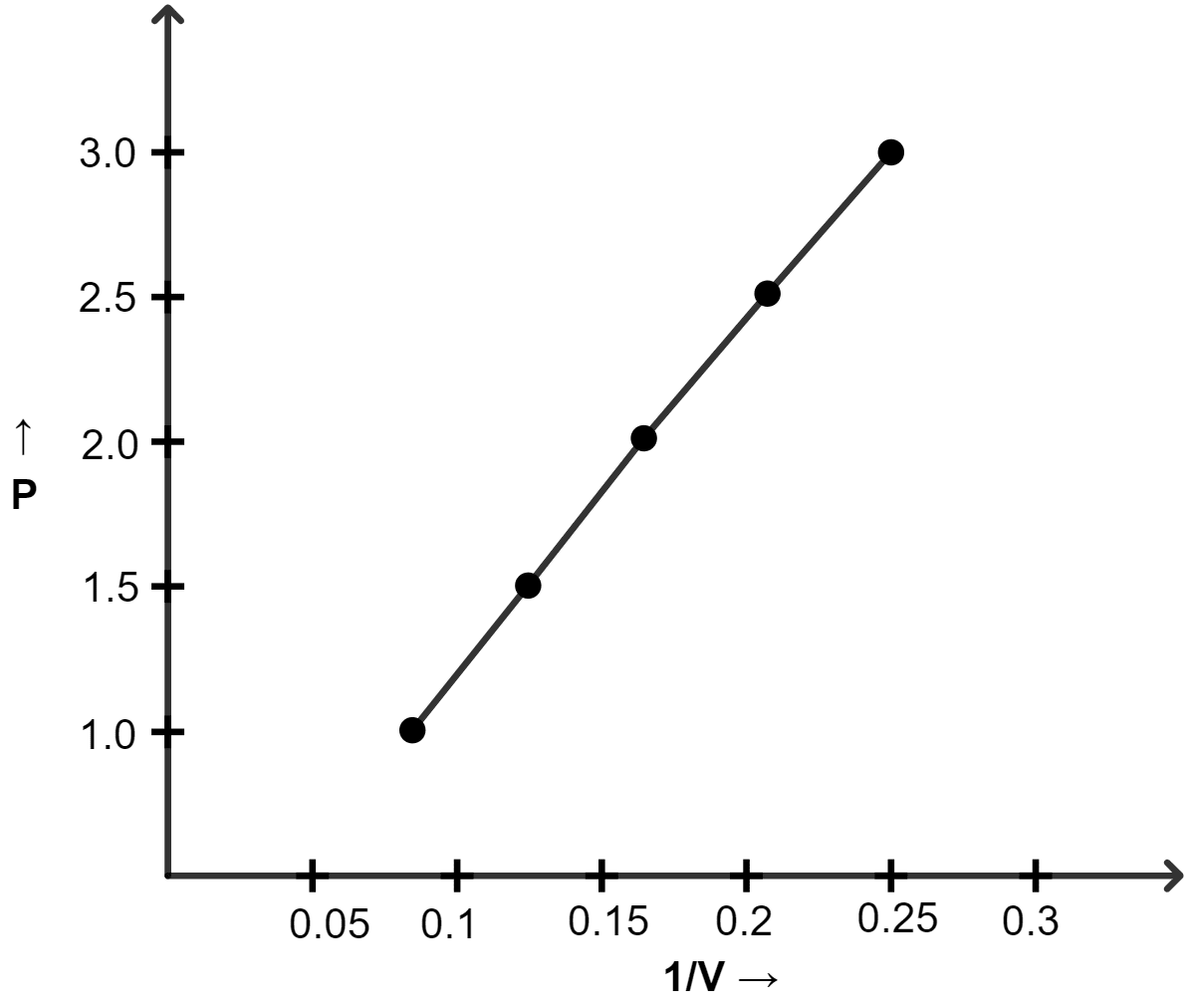
Chapter 7 Study Of Gas Laws Selina Solutions Concise Chemistry Class

According To Boyle s Law The Shape Of The Graph Of Pressure Vs The
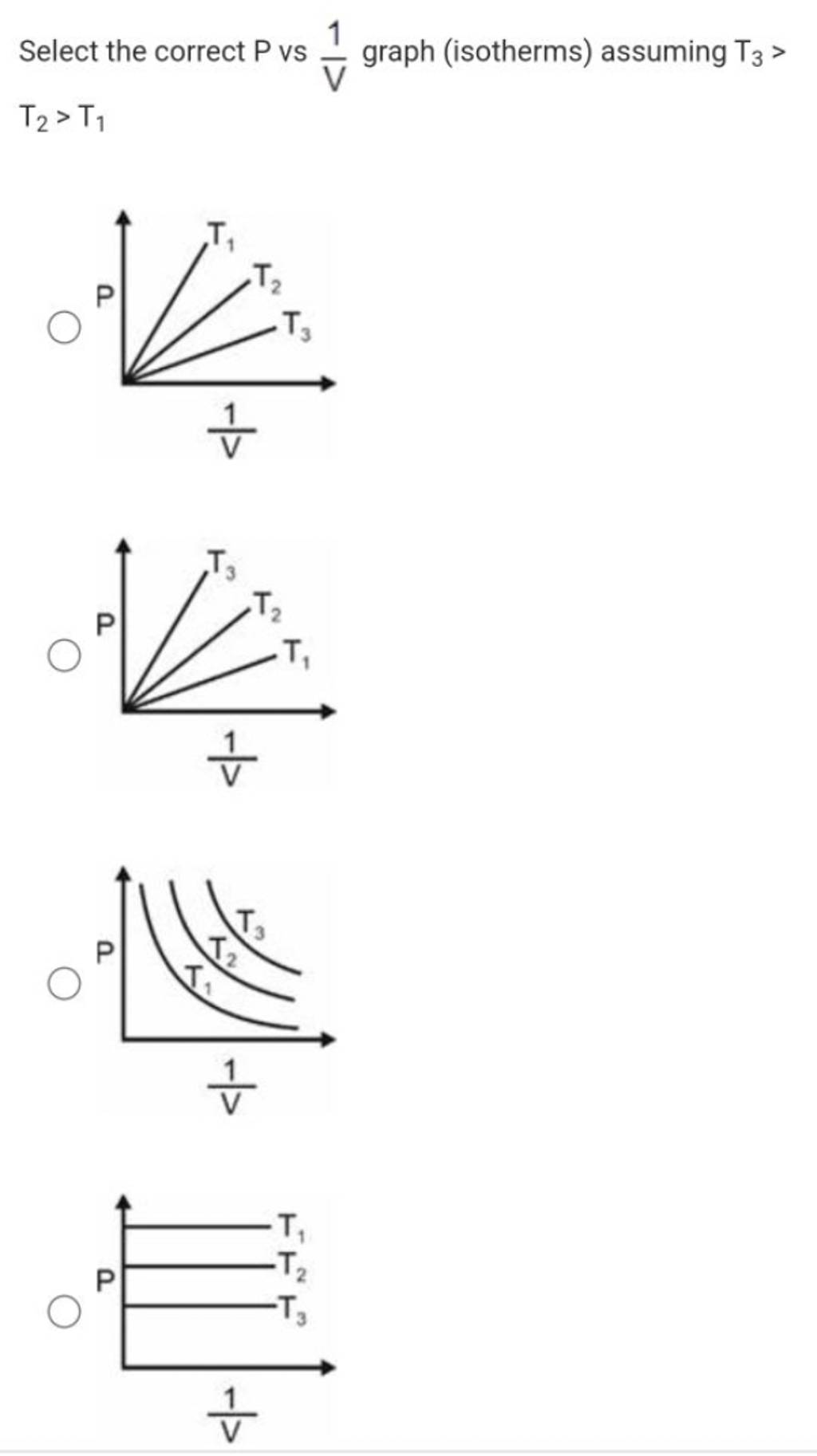
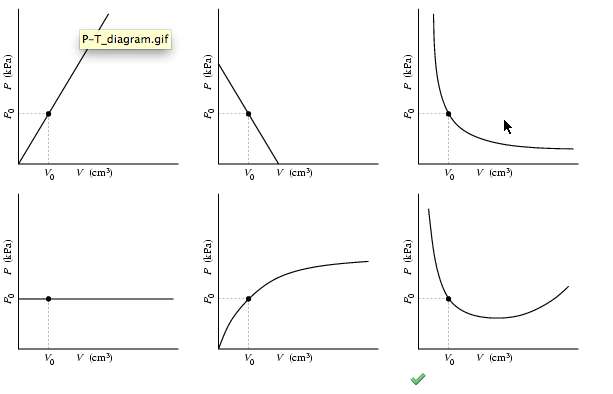
Select The Correct P Vs V1 Graph isotherms Assuming T3 T2 T1
What Is The Slope Of LogP Vs LogV Graph At Constant Temperature
What Is The Slope Of LogP Vs LogV Graph At Constant Temperature

Boyle s Law May Be Expressed As

The Behavior Of Gases Boyle s And Charles Laws Name Chegg

8 2 Relating Pressure Volume Amount And Temperature The Ideal Gas
P Vs 1 V Graph - P is the pressure V is the volume n is the number of moles of amount of substance of gas R is the ideal or universal gas constant T is the temperature of the gas Since temperature is kept constant the RHS of the equation is a constant PV Constant As such the graph of PV against P should be a straight line parallel to the P axis