Post Market Study Medical Device ID POST ID PUT GET user 123 REST
Nature Communications NC NC
Post Market Study Medical Device

Post Market Study Medical Device
https://i.ytimg.com/vi/FAobG-wAPiY/maxresdefault.jpg

Regulatory Affairs PQE Group US
https://www2.pqegroup.com/us/wp-content/uploads/sites/15/2022/08/regulatory-affairs-md.png
Regulatory Affairs PQE Group US
https://www2.pqegroup.com/us/wp-content/uploads/sites/15/2022/08/regulatory-affairs-intelligence.png
Alguns ensinam que POST para enviar dados para cria o de algo e que PUT para atualizar mas achei mal explicado Ent o afinal qual a diferen a entre o m todo PUT 11
Zotero Scholaread bug C ProgramData NVIDIA Corporation NetService NVIDIA C Program Files NVIDIA Corporation Installer2
More picture related to Post Market Study Medical Device

Medical Device Blogs Elexes
https://www.elexes.com/wp-content/uploads/2023/08/eCopy-Medical-Device-Submission-1.png

The 3 Stages For Medical Device Clinical Investigation Explained
https://diagramresearch.com/content/uploads/2022/01/3-Stages-For-Medical-Device-Clinical-Investigation.png

What Is Post Market Post Marketing Surveillance ArborMetrix
https://global-uploads.webflow.com/5f7097e89d18be3b8d3d2f9d/5f944be262f66dfd9920fe64_Key-Goals-of-Post-Market-Surveillance.png
Zotero Deepl API Deepl API 20w 20w rGWwPG UVM EI SCI
[desc-10] [desc-11]

Post market Surveillance Plan
http://medicaldeviceacademy.com/wp-content/uploads/Screenshot-2015-12-15-at-6.18.57-AM.png

Statistical Report Eclevar MedTech Medical Device CRO
https://www.eclevarmedtech.com/wp-content/uploads/2022/10/Statistical-report-of-pmcf-eclevar-medtech-1024x1024.png


https://www.zhihu.com › question
Nature Communications NC NC

EU Medical Device PMS Report And PSUR Specculo

Post market Surveillance Plan
Post Market Surveillance Procedure

FDA Premarket Approval FDA PMA Submission Fang Consulting
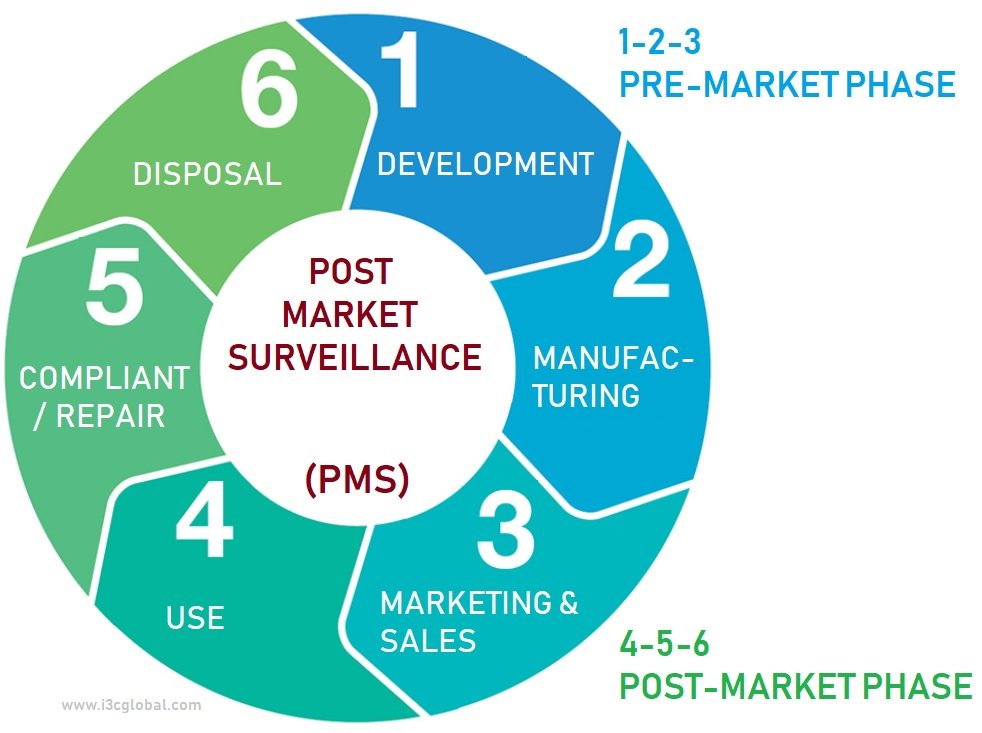
Post Market Surveillance Medical Device Procedure I3CGLOBAL

Linking Intermacs To The World Ppt Download

Linking Intermacs To The World Ppt Download

Emergo By UL
Medical Device Feasibility Study The Definitive Guide
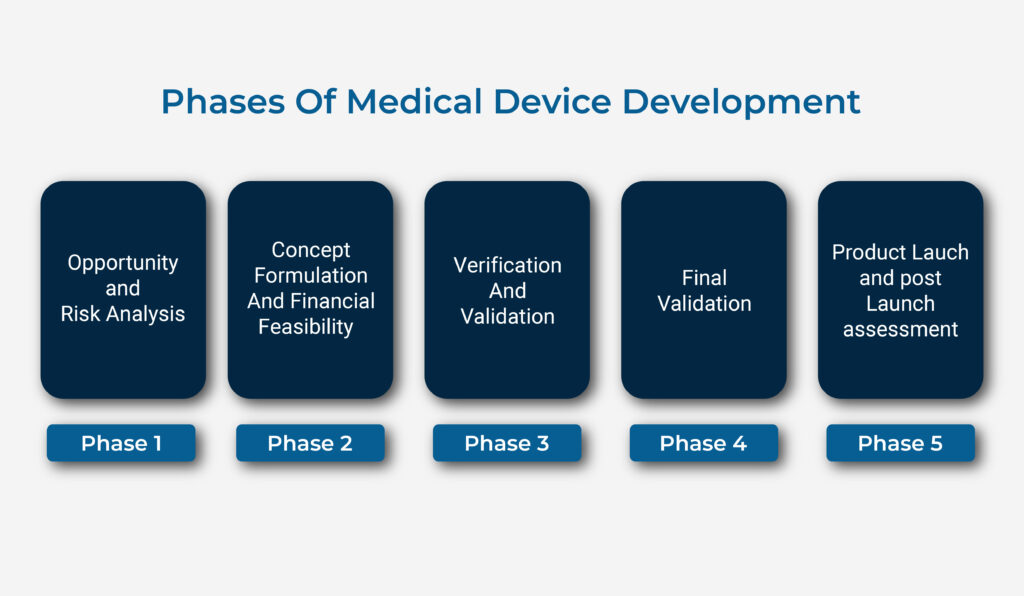
Medical Device Product Development Process
Post Market Study Medical Device - [desc-14]