What Is A Group Number There are total 18 different groups in Periodic table Let me explain each of these groups in short Alkali metals group is the very first group group 1 on the periodic table The elements included in the Alkali metals group are For detailed information on Alkali metals read the Ultimate guide on Alkali metals of periodic table
The vertical columns of the periodic table are called groups According to the IUPAC system of naming groups there are 18 groups with the group number ranging from 1 to 18 The elements in each group have the same number of valence electrons and hence have similar chemical properties determined by the outermost electrons What does the Group number and Period number tell you The group number tells you the total number of electrons present in the outermost orbit of an atom In other words group number tells you the number of valence electrons of an atom For example Let us consider group 1 of the Periodic table
What Is A Group Number

What Is A Group Number
https://i.ytimg.com/vi/y1VuY_IN8jg/maxresdefault.jpg

How To Find Group Number And Period Number Of An Element Periodic
https://i.ytimg.com/vi/ASB7LZ-fI1M/maxresdefault.jpg
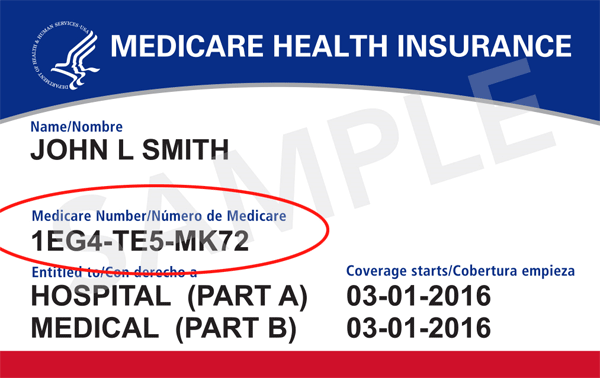
BCBS Group Medicare Plan
https://bcbsmgroupmedicareplan.com/img/medicare_card_number_circled.png
The main groups are numbered from 1 to 7 going from left to right and the last group on the right is Group 0 the block in between Group 2 and Group 3 is where the transition metals are A group is a vertical column down the periodic table while a period is a horizontal row across the table Both groups and periods reflect the organization of electrons in atoms Element atomic number increases as you move down a group from top to bottom or across a period from left to right
A group is a vertical column of the periodic table based on the organization of the outer shell electrons There are a total of 18 groups There are two different numbering systems that are commonly used to designate groups and you should be familiar with both The group number represent the valence electron s present in the elements belonging to a specific group The chemical physical properties of some elements belonging to the same group are identical Examples Group 1 is known as Alkali metals Group 2 is known as Alkaline earth metals and Group 17 is known as Halogens
More picture related to What Is A Group Number

Introduction The Periodic Table
http://mehdiperiodictable.weebly.com/uploads/2/6/4/5/26457355/group-and-period-numbers_orig.jpg

Neopteron Camouflage MHWilds
https://monsterhunterwiki.org/images/f/fe/MHWilds-Logo.png?version=2ab6ff7e09cd6a3c4f4603a79bcd9f1b
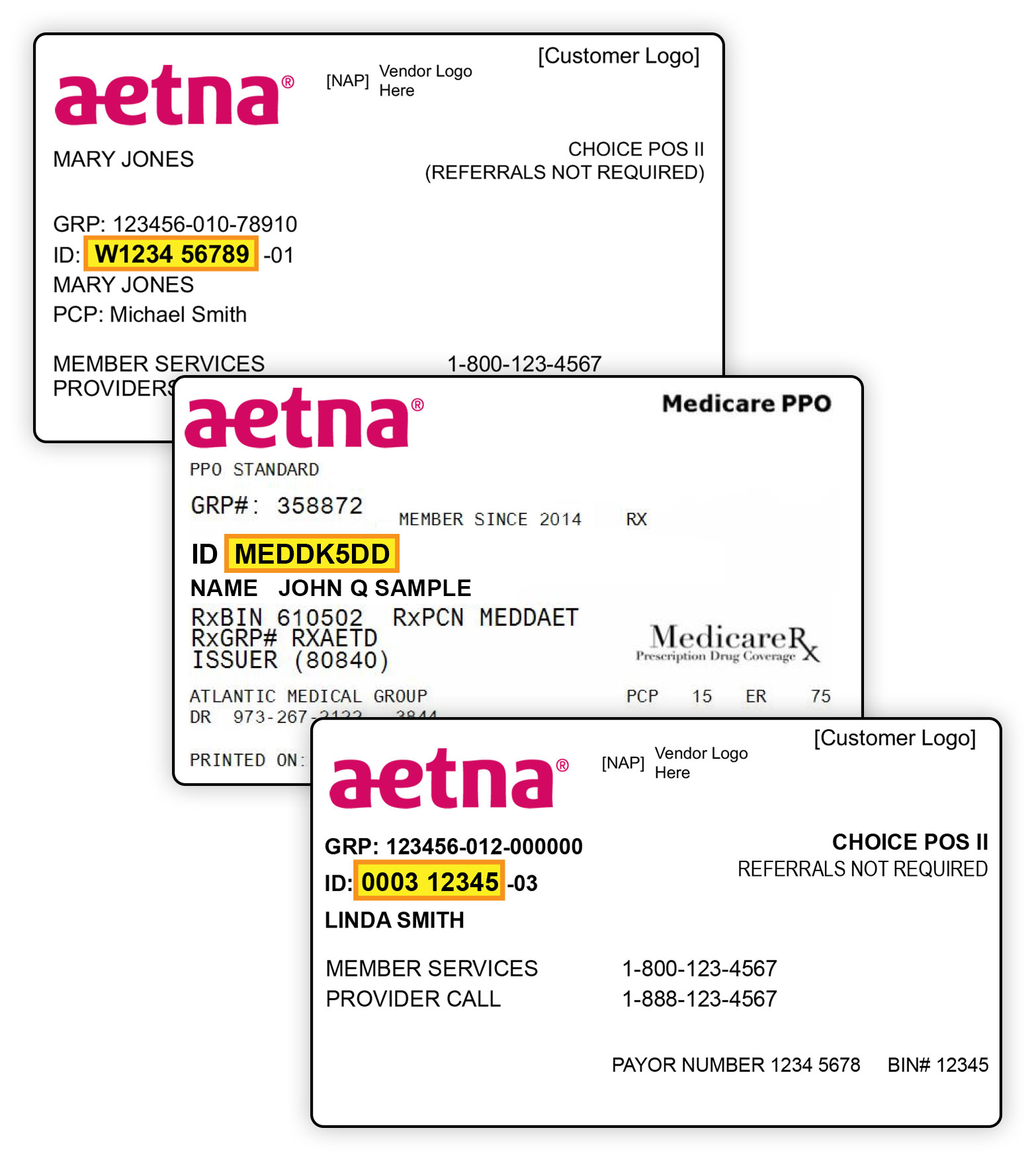
Aetna Group Number Tube Wife Blowjob
https://www.aetna.com/AccountManagerAppConfig/commonV2/includes/memberRegv2/images/id_card_highlight.jpg
Groups The vertical column of the periodic table that signifies the number of valence electrons in an element Periods The horizontal rows in the periodic table that signify the number of electron shells in an element Explain how elements are organized into the periodic table Locate elements based on their atomic number Identify the period and group in which elements are found Classify elements as metals non metals or metalloids
[desc-10] [desc-11]
Deloitte 401k Match
https://sqy7rm.media.zestyio.com/What-is-a-401-k--Match-and-How-Does-It-Work--.jpg
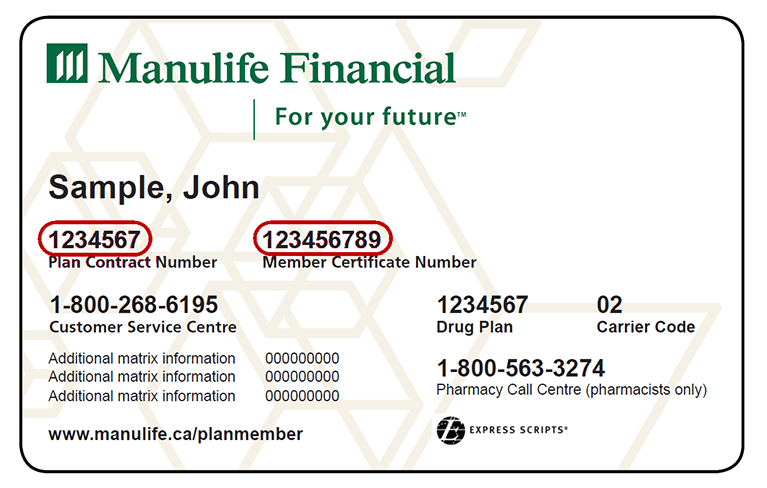
Group Benefits Mobile Site Login
https://gbpmfmo.manulife.com/MobileUIAssets/img/en-CA/benefit-card-en.png

https://periodictableguide.com › periodic-table-groups
There are total 18 different groups in Periodic table Let me explain each of these groups in short Alkali metals group is the very first group group 1 on the periodic table The elements included in the Alkali metals group are For detailed information on Alkali metals read the Ultimate guide on Alkali metals of periodic table

https://www.chemistrylearner.com › the-periodic...
The vertical columns of the periodic table are called groups According to the IUPAC system of naming groups there are 18 groups with the group number ranging from 1 to 18 The elements in each group have the same number of valence electrons and hence have similar chemical properties determined by the outermost electrons

Forming A Group For Given Numbers Worksheets Math Worksheets
Deloitte 401k Match

Member ID Card

Form A Group For The Given Numbers Math Worksheets MathsDiary
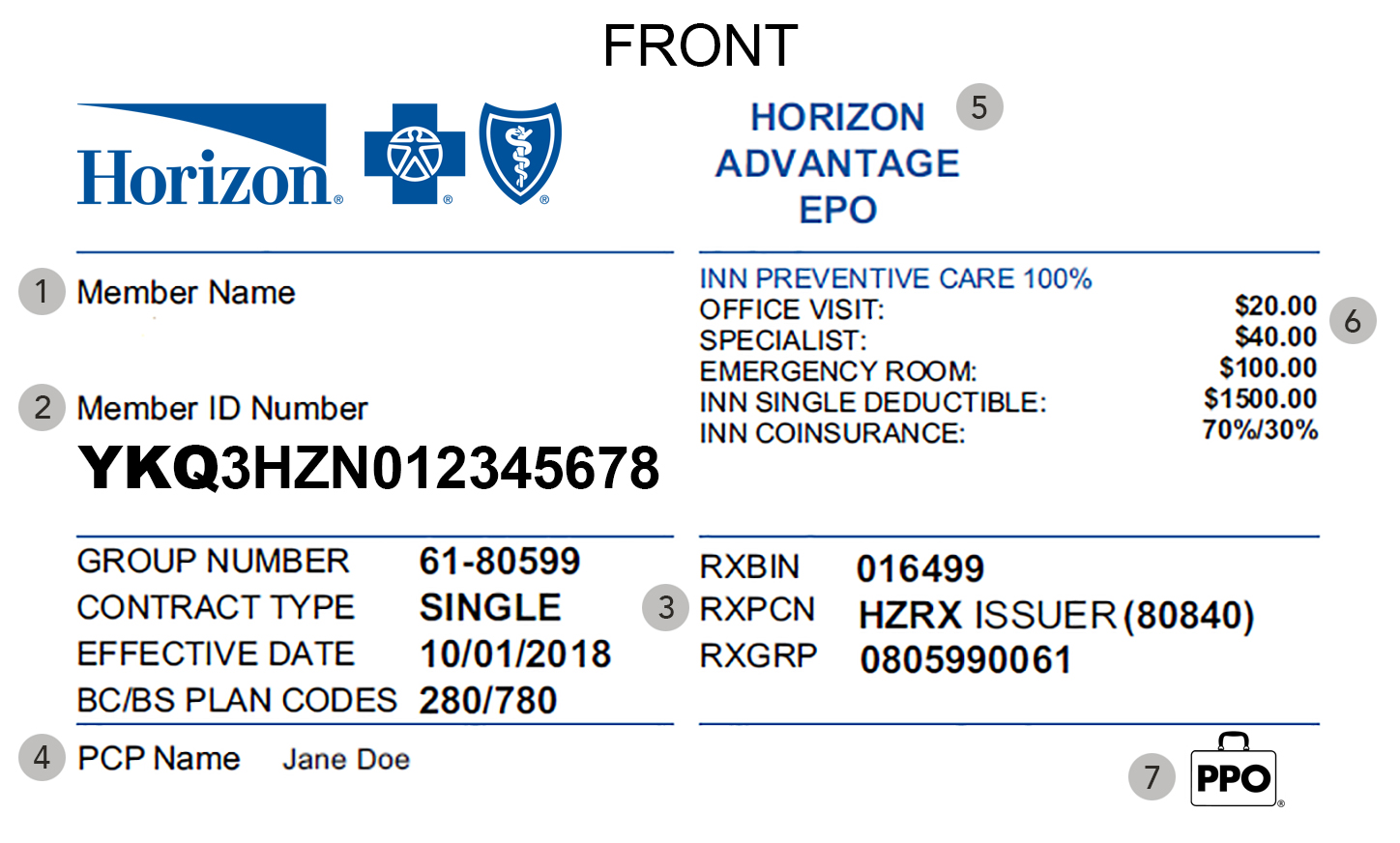
State Of Nj Health Benefits Id Card Mamiihondenk

Periodic Table Definition Groups Britannica

Periodic Table Definition Groups Britannica
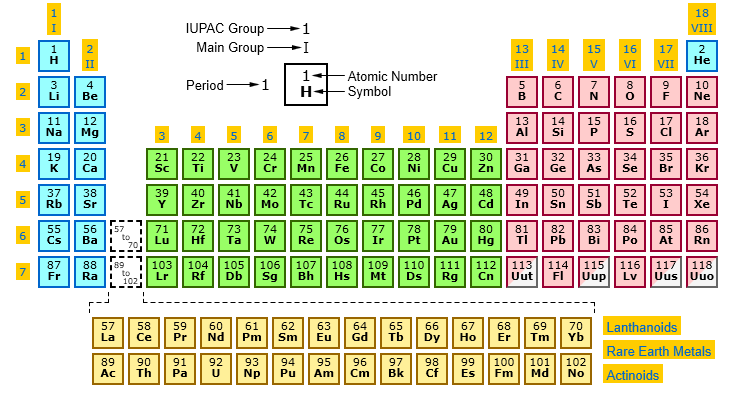
Main groups highlighted periodic table

Periodic Table Periods Groups And Families

How To Find Cbfinaid Id
What Is A Group Number - A group is a vertical column of the periodic table based on the organization of the outer shell electrons There are a total of 18 groups There are two different numbering systems that are commonly used to designate groups and you should be familiar with both