What Is Analytical Method Transfer Analytical method transfer is a verification process which has much in common with the validation activities described in EU GMP Annex 15 and the guidance it contains provides a useful
Learn about method transfer and its importance in analytical laboratories Explore the key steps and challenges involved in transferring analytical methods The transfer of an analytical method is defined as the documented process that qualifies a laboratory receiving laboratory to use an analytical method that originated in another laboratory transferring laboratory whether that is internal or external to the receiving laboratory
What Is Analytical Method Transfer

What Is Analytical Method Transfer
https://i.ytimg.com/vi/CsmouUOnBos/maxresdefault.jpg

Analytical Method Development Validation YouTube
https://i.ytimg.com/vi/3lQ5W4CyklU/maxresdefault.jpg

SHAK DEM R On Twitter erefsizsiniz Haysiyet Yoksunlar pl n zde
https://pbs.twimg.com/media/FwMPkzvWYA4Jop8.jpg
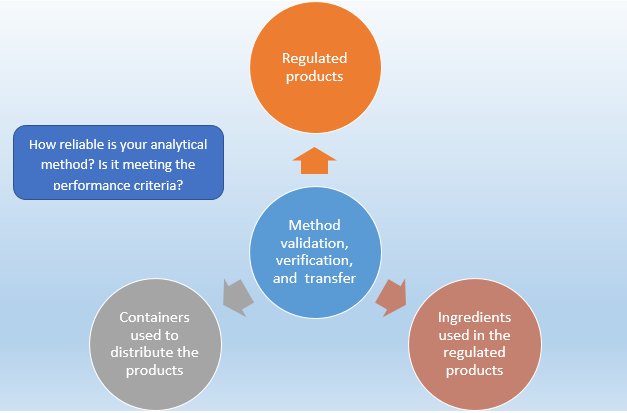
In analytical method validation all validation parameters are performed to see the suitability of the method for intended use whereas in analytical method transfer selected parameters like reproducibility specificity and quantitation limit are performed In an analytical method transfer the receiving laboratory becomes qualified to perform the methods being transferred One of the most important things in this process is the transfer of the technical and scientific knowledge of the method from the sending laboratory to the receiving laboratory
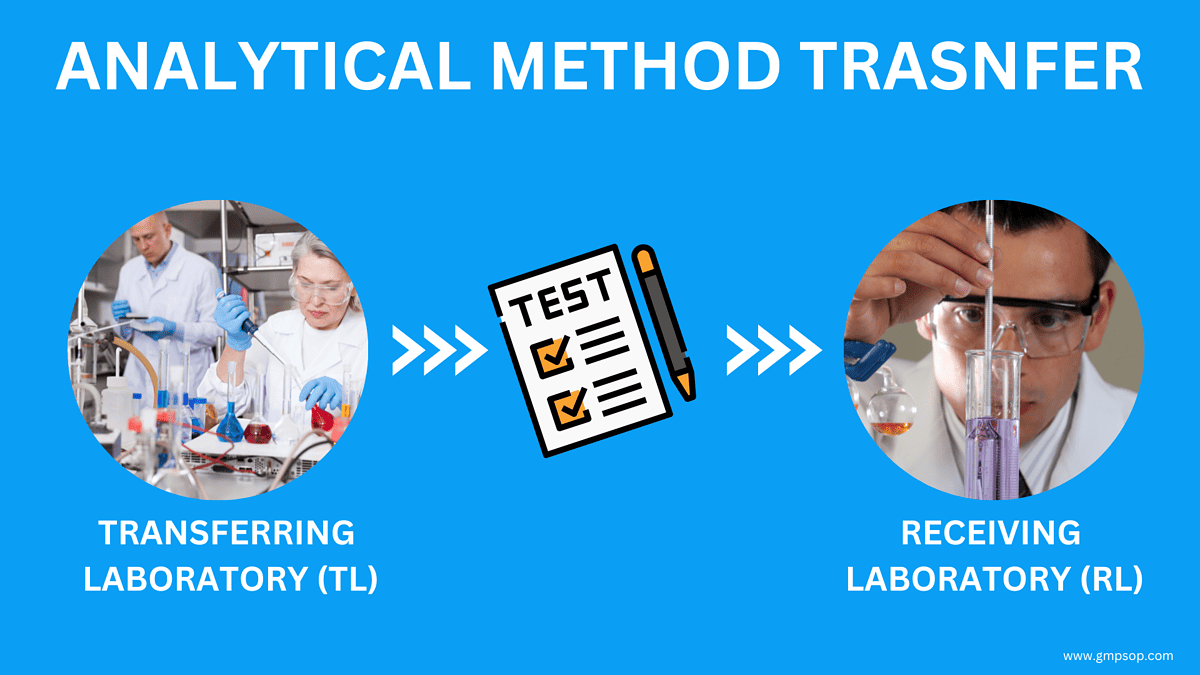
The transfer of an analytical procedure is the documented process that qualifies a laboratory to use the method that originated in another laboratory therefore ensuring that the receiving unit has the procedural knowledge and ability to perform the procedure as intended What is Analytical Method Transfer Analytical method transfer is the process that qualifies a receiving lab to use a method that originated in another lab The receiving lab in question may be an internal or external lab
More picture related to What Is Analytical Method Transfer

What Precautions One Must Take During Analytical Method Transfer YouTube
https://i.ytimg.com/vi/IakOYgziHOM/maxresdefault.jpg

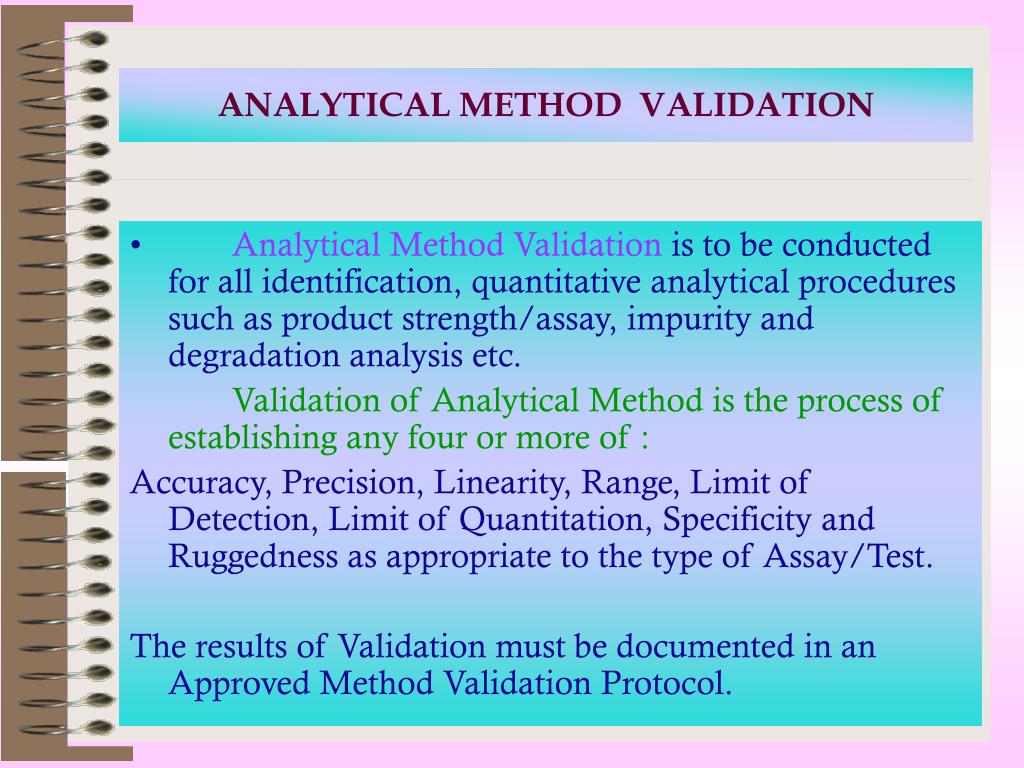
VALIDATION OF ANALYTICAL METHOD Method Validation Validation Of An
https://i.ytimg.com/vi/Ubly1dmEF8w/maxresdefault.jpg

What Is Analytical Method Validation YouTube
https://i.ytimg.com/vi/QIU_0XM6Ru0/maxresdefault.jpg
Planning for an upcoming Tech Transfer Review our simple checklist to see if you are prepared to conduct an analytical method transfer The transfer of analytical procedures TAP also referred to as method transfer is the documented process that qualifies a laboratory the receiving unit to use an analytical test procedure that originated in another laboratory the transferring unit thus ensuring that the receiving unit has the procedural knowledge and ability to perform
[desc-10] [desc-11]

Recap Analytical Versus Numerical Solutions To ODEs YouTube
https://i.ytimg.com/vi/E5k08Y4xzyc/maxresdefault.jpg
Analytical Method Validation For Quality Assurance And 58 OFF
https://static.complianceonline.com/images/articles/Analytical_Method_Validation.png

https://mhrainspectorate.blog.gov.uk › transfer-of-analytical-methods
Analytical method transfer is a verification process which has much in common with the validation activities described in EU GMP Annex 15 and the guidance it contains provides a useful

https://biotech.com › what-is-method-transfer-and-why-is-it-important
Learn about method transfer and its importance in analytical laboratories Explore the key steps and challenges involved in transferring analytical methods
GMPSOP Blog Good Manufacturing Practice

Recap Analytical Versus Numerical Solutions To ODEs YouTube

Analytics Report Sample

Analytical Method Transfer Assay Method Transfer Services NorthEast
Analytical Method Validation Vrogue co

Analytical Method Validation Vrogue co

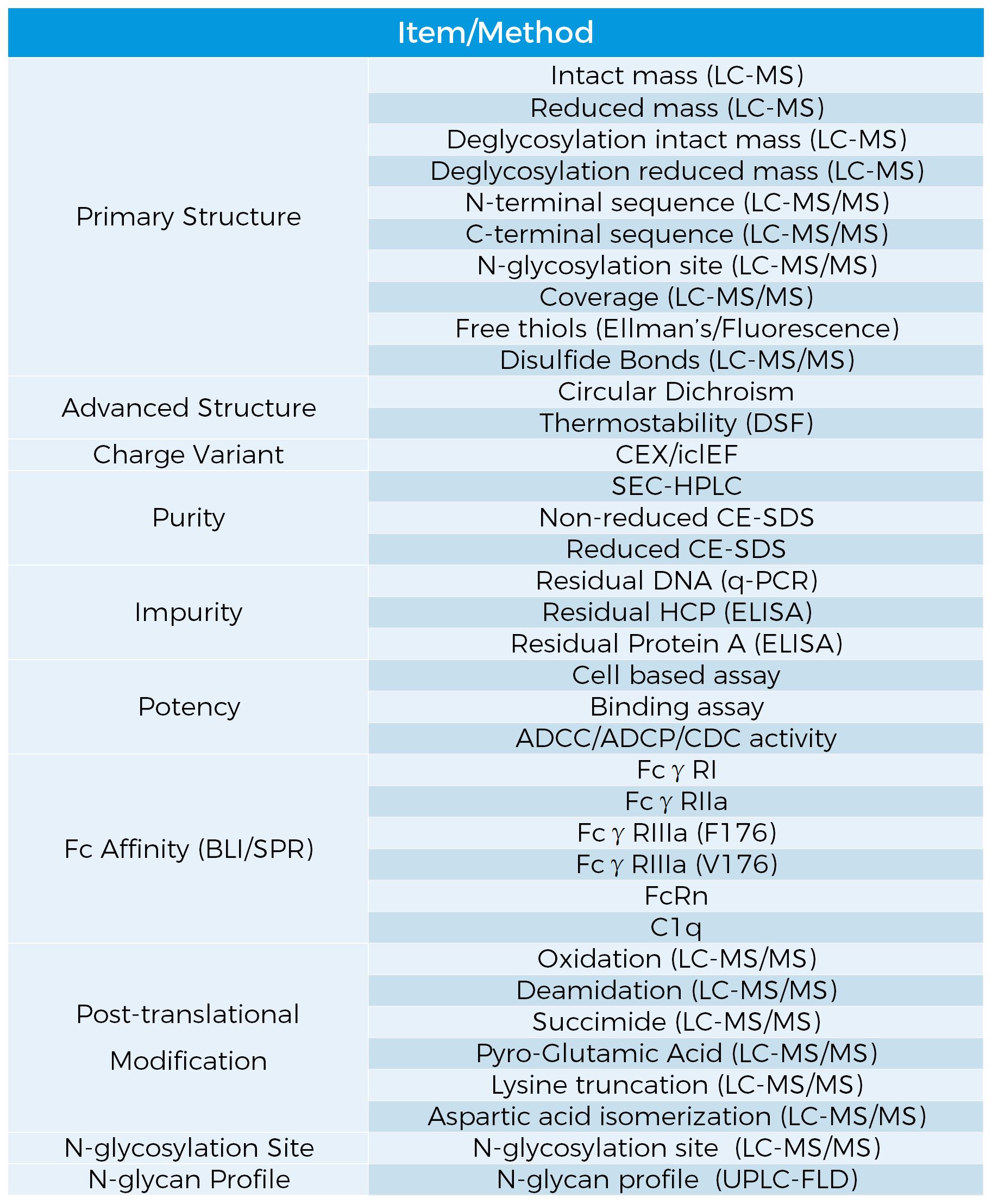
Analytical Methods

45 Analysis Examples 2025

What Is Analytical Method Transfer - In analytical method validation all validation parameters are performed to see the suitability of the method for intended use whereas in analytical method transfer selected parameters like reproducibility specificity and quantitation limit are performed