What Is Diffusion In Chemistry Diffusion is the movement of molecules from a region of higher concentration to a region of lower concentration down the concentration gradient Learn ab
Diffusion is the process by which particles of one substance spread out through the particles of another substance Diffusion is how smells spread out through the Spreading out and Mixing of one substance with other is called Diffusion It is fastest in gases and slowest in solids The smell of cooked food reaches our nose because of
What Is Diffusion In Chemistry

What Is Diffusion In Chemistry
https://nayturr.com/wp-content/uploads/2020/07/liquid-diffusion-july012020-min.jpg

Diffusion Vector Illustration Labeled Educational Particles Mixing
https://i.pinimg.com/originals/a5/68/d2/a568d2968641c6a6db2c49be90790aa2.jpg
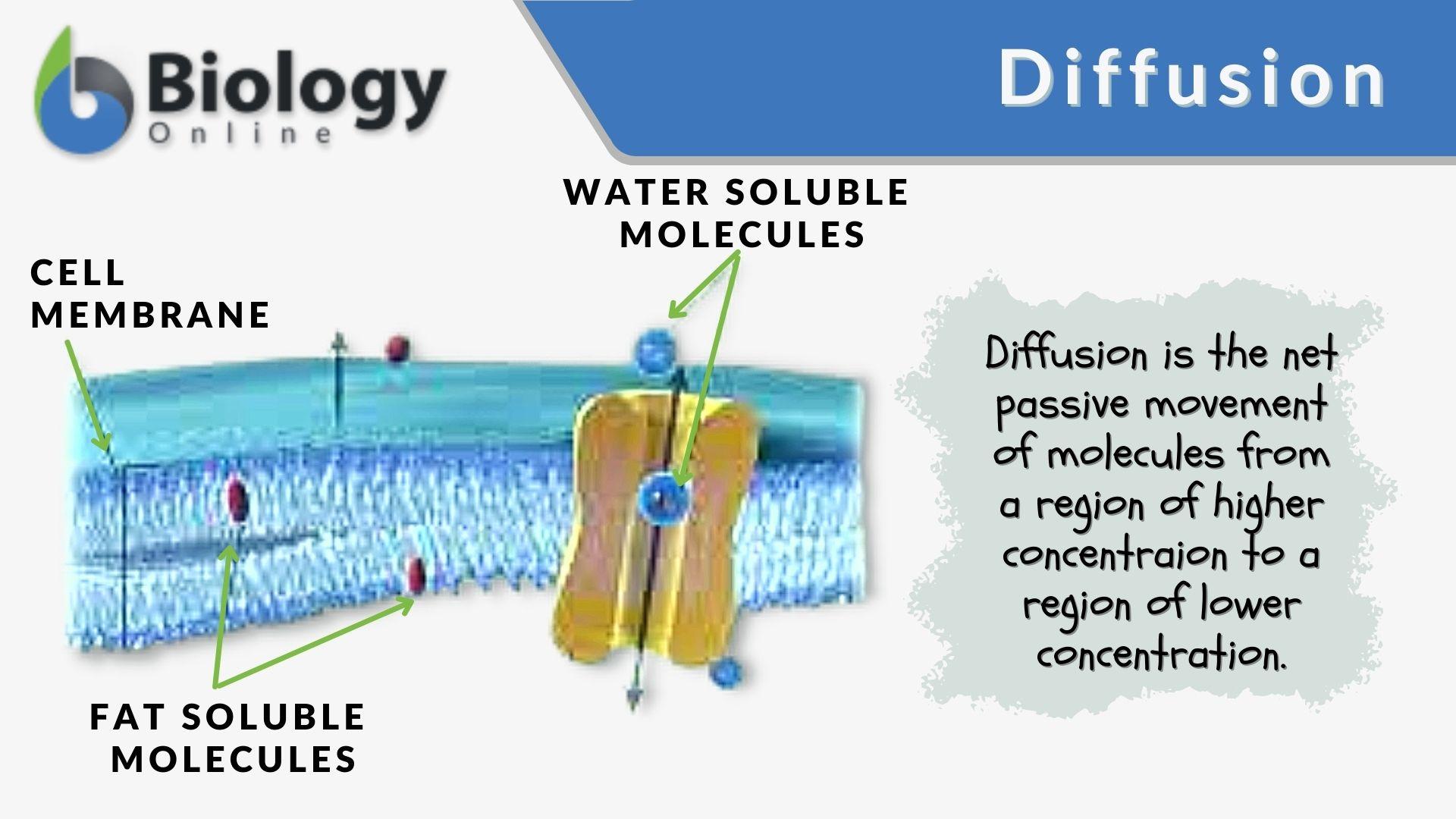
Facilitated Diffusion Examples
https://www.biologyonline.com/wp-content/uploads/2019/11/diffusion-definition-and-example.jpg
Diffusion Diffusion occurs in gases and liquids due to the random motion of their particles It is where particles move from an area of high concentration to an area of low In chemistry diffusion influences the rate of chemical reactions Different substances whether solids liquids or gases diffuse at different rates affecting how quickly
Diffusion is a fundamental process involving the movement of particles such as atoms ions or molecules from an area of higher concentration to one of lower concentration Simple diffusion and osmosis do not involve transport proteins Facilitated diffusion requires the assistance of proteins Diffusion is the movement of molecules from an area of high concentration of the molecules to an area
More picture related to What Is Diffusion In Chemistry
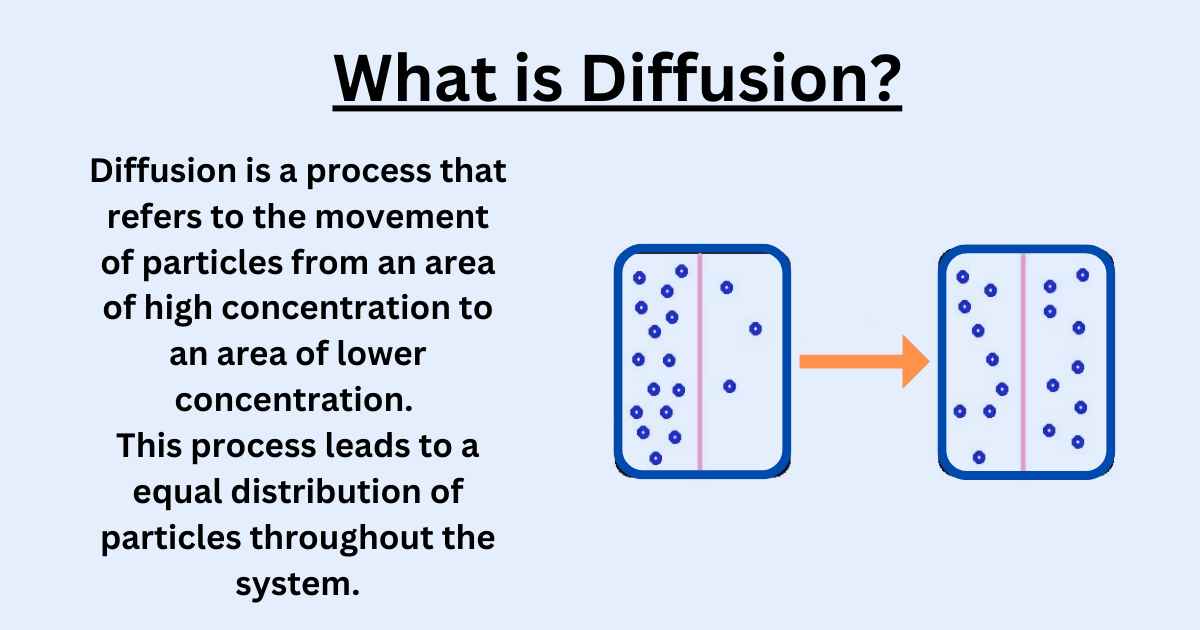
Diffusion Explained Types Examples And Factors
https://eduinput.com/wp-content/uploads/2023/05/What-is-Diffusion-image.jpg

2 1 1 The Particle Theory Of Matter Revision my
http://spmchemistry.blog.onlinetuition.com.my/wp-content/uploads/sites/2/2014/12/diffusion-08.png
Diffusion 1 8K Plays Quizizz
https://quizizz.com/media/resource/gs/quizizz-media/quizzes/73e6b158-5bc0-4b0a-b242-53a5aaed22e8
The movement of gases like carbon dioxide and oxygen from a region of high concentration to a region of low concentration through a semi permeable membrane is known as diffusion Thus Learn the difference between osmosis and diffusion two types of mass transport processes in chemistry and biology Osmosis is the movement of solvent across a semipermeable membrane while diffusion is the movement
Learn about diffusion and dilution for IGCSE Chemistry with experiments illustrating the kinetic theory of matter how particles move in gases and liquids Diffusion is a process of moving atoms in a material from a high concentrated area to low concentrated area The material can be solid liquid or gas diffusion is widely used in
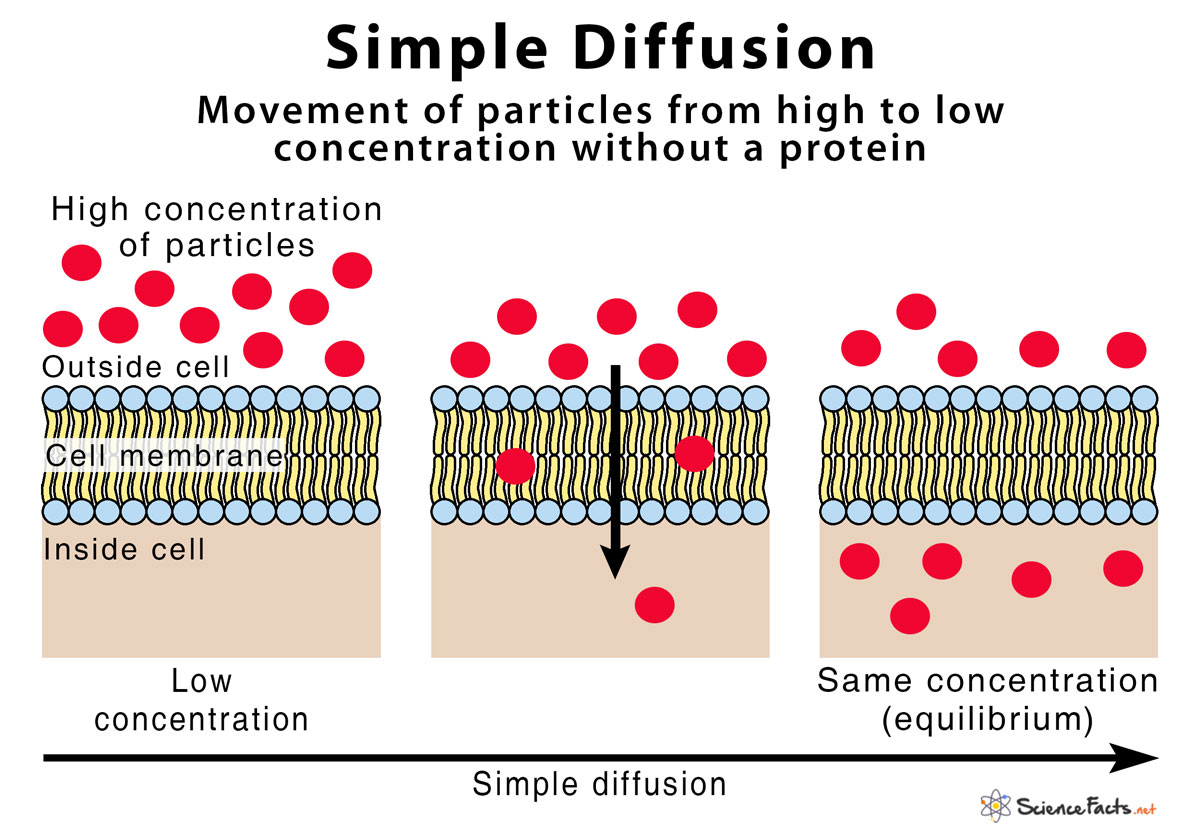
Simple Diffusion Definition With Examples And Diagram
https://www.sciencefacts.net/wp-content/uploads/2020/02/Simple-Diffusion.jpg

What Is Diffusion Simple Diffusion Facilitated Diffusion Biology
https://i.ytimg.com/vi/h9KVjtprRJg/maxresdefault.jpg

https://byjus.com › biology › diffusion
Diffusion is the movement of molecules from a region of higher concentration to a region of lower concentration down the concentration gradient Learn ab

https://www.bbc.co.uk › bitesize › articles
Diffusion is the process by which particles of one substance spread out through the particles of another substance Diffusion is how smells spread out through the

Diffusion I Chemistry Visionlearning

Simple Diffusion Definition With Examples And Diagram

What Is Facilitated Diffusion Definition Principle Types Importance
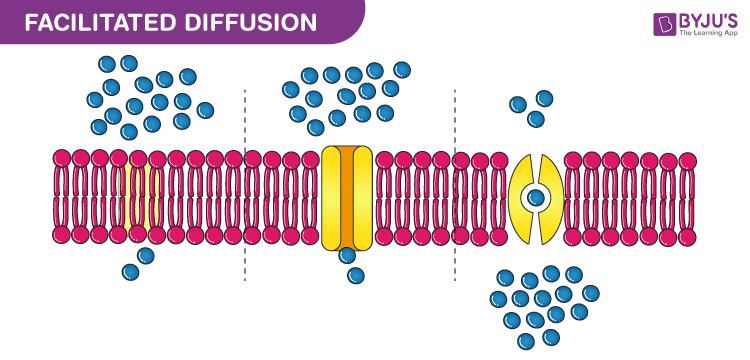
Definition Of Diffusion In Chemistry

Definition Of Diffusion Chemistry

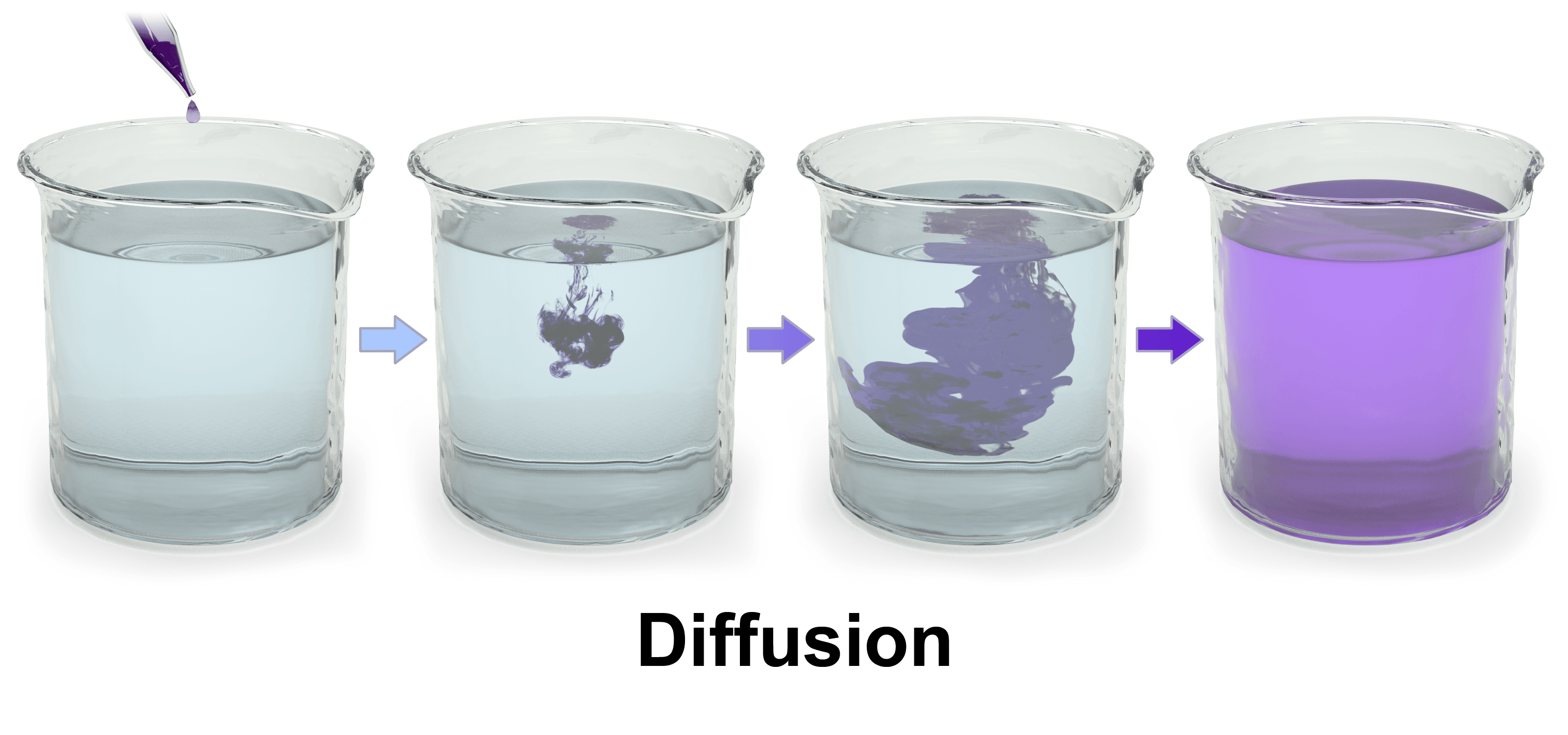
Diffusion Demonstration What Is Diffusion

Diffusion Demonstration What Is Diffusion
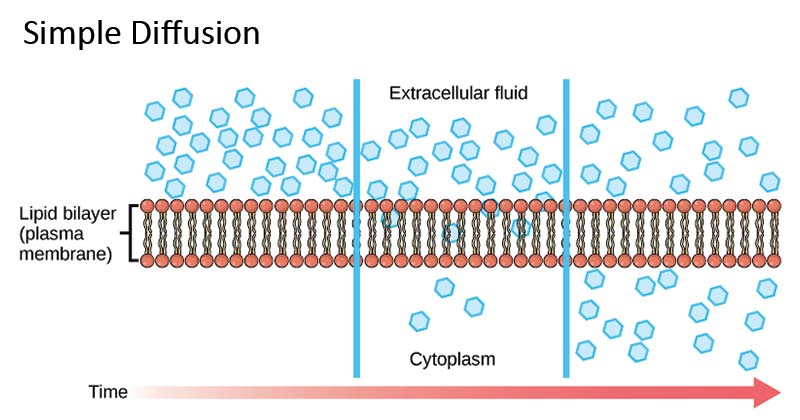
Definition Of Diffusion In Chemistry
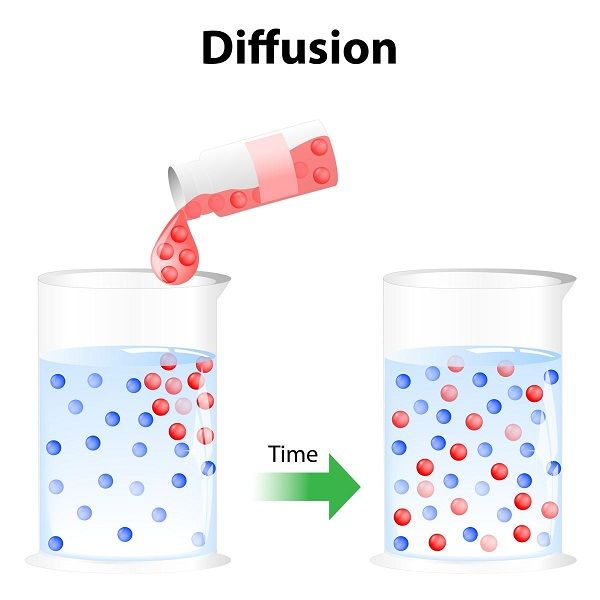
10 Examples Of Diffusion In Everyday Life StudiousGuy
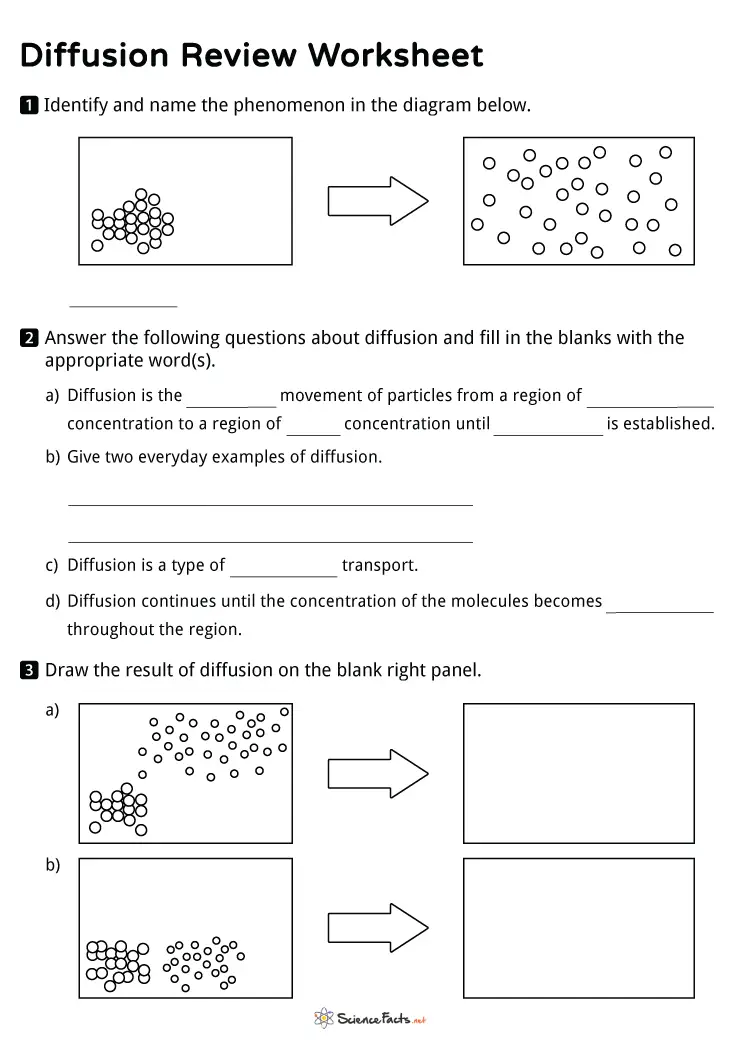
Diffusion Worksheets Free Printables
What Is Diffusion In Chemistry - In chemistry diffusion influences the rate of chemical reactions Different substances whether solids liquids or gases diffuse at different rates affecting how quickly