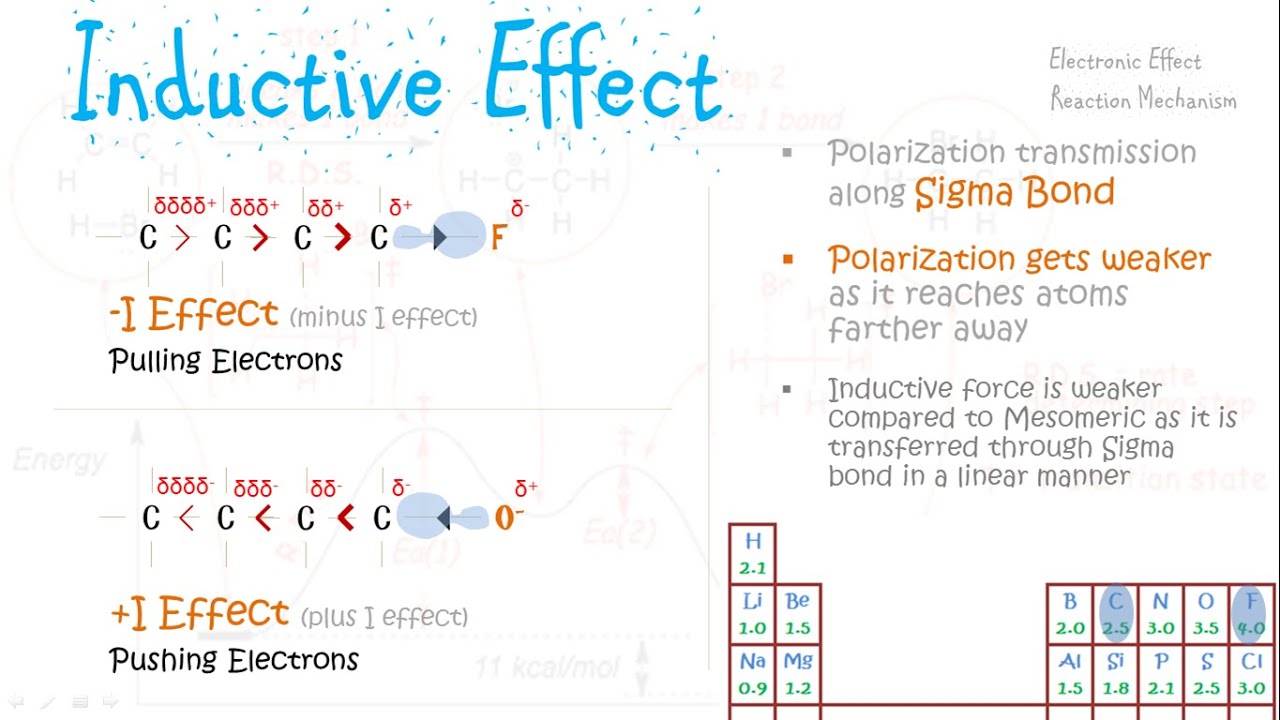
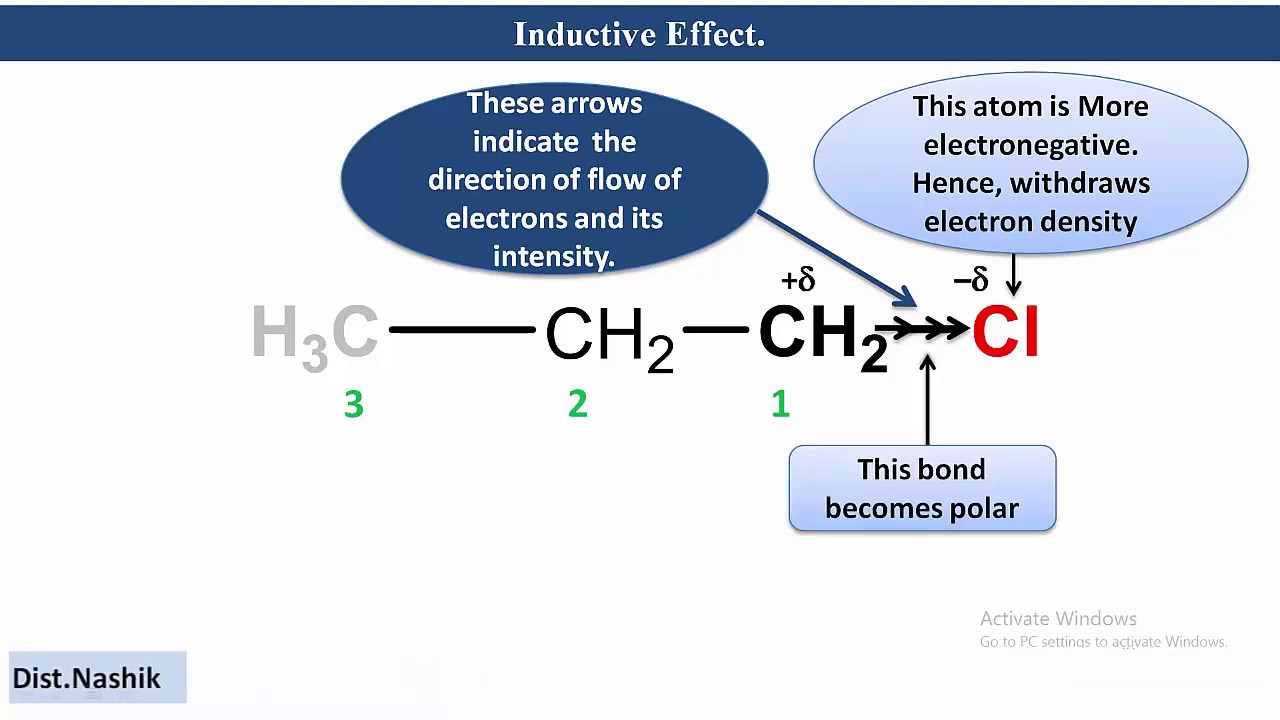
What Is Negative Inductive Effect An electron withdrawing property of a group or atom is known as its negative inductive effect I The symbol for this is I When an electronegative atom such as a halogen is introduced into a chain of atoms typically carbon atoms the unequal distribution of electrons results in the transmission of a positive charge through the chain
Negative inductive effect can be observed in electron withdrawing atoms or groups that tends to withdraw electron density from a neighboring atom through sigma bonds Common examples of electronegative atoms are oxygen fluorine chlorine and bromine In this process the electronegative atom gets partial negative charge while the other atom gets partial positive charge Thus induced polarity is transmitted through the sigma bonds in the molecule by creating a permanent dipole This phenomenon is referred to as inductive effect
What Is Negative Inductive Effect
What Is Negative Inductive Effect
https://i.ytimg.com/vi/nlpRcY4nHpU/maxresdefault.jpg

Inductive Effect Organic Chemistry YouTube
https://i.ytimg.com/vi/-JrFIB3DYzk/maxresdefault.jpg
What Is Inductive Effect YouTube
https://i.ytimg.com/vi/QzrMCRNO8wQ/maxresdefault.jpg
Negative Inductive Effect Characterises atoms or groups in a molecule that pull or accept electrons from other atoms or groups due to their enhanced electronegativity It helps stabilise carbocations and increase acidity of compounds 1 Electron pulling negative inductive I effect If the atom or the group of atoms pulls the bond electrons then such an effect is called a negative Inductive effect written as I effect For example in CH 3 CH 2 CH 2 Cl electronegative Chlorine withdraws the electron density from the carbon carbon bonds
The two types of inductive effects are Negative Inductive Effect I Electron withdrawing groups pull electron density away from the rest of the molecule Positive Inductive Effect I Electron donating groups push electron density toward the rest of the molecule When electronegative atoms or groups such as halogens fluorine chlorine etc displace the electron density towards themselves they exhibit a negative inductive effect One of the most important applications of the inductive effect in organic chemistry relates
More picture related to What Is Negative Inductive Effect

Calculate Inductive And Capacitive Reactance YouTube
https://i.ytimg.com/vi/BPq-am7DkNM/maxresdefault.jpg

Comparing Acidities Inductive Effects YouTube
https://i.ytimg.com/vi/lUXXV12JjfU/maxresdefault.jpg

4 Negative Inductive Effect Explanation YouTube
https://i.ytimg.com/vi/to-IglcQ5n4/maxresdefault.jpg
Negative Inductive Effect I Effect Conversely groups or atoms that withdraw electron density through sigma bonds exhibit a I effect These electron withdrawing groups increase the positive charge on adjacent atoms stabilizing anions Negative Inductive Effect I When an electronegative atom such as a halogen is added to a chain of atoms usually carbon atoms the unequal sharing of electrons produces a positive charge that is carried through the chain
[desc-10] [desc-11]

14 Differences Between Inductive Effect And Electromeric Effect YouTube
https://i.ytimg.com/vi/tHnLIBF9qwU/maxresdefault.jpg
Inductive Effect Definition Types Examples Applications IIT JEE NEET
https://i.ytimg.com/vi/JbIjJp7hgKk/maxresdefault.jpg

https://scienceinfo.com › inductive-effect-types-uses-stability
An electron withdrawing property of a group or atom is known as its negative inductive effect I The symbol for this is I When an electronegative atom such as a halogen is introduced into a chain of atoms typically carbon atoms the unequal distribution of electrons results in the transmission of a positive charge through the chain

https://www.geeksforgeeks.org › inductive-effect
Negative inductive effect can be observed in electron withdrawing atoms or groups that tends to withdraw electron density from a neighboring atom through sigma bonds Common examples of electronegative atoms are oxygen fluorine chlorine and bromine

5 Positive Inductive Effect Explanation And Example Of Inductive

14 Differences Between Inductive Effect And Electromeric Effect YouTube

Inductive Effect positive And Negative Inductive Effect Acidity And

Inductive Effect I And I Effect Electronic Displacement Basic

Order Of Negative Inductive Effect Group Tricks Mnemonics Explained

Negative Inductive Effect Is Shown By Brainly in

Negative Inductive Effect Is Shown By Brainly in

PLZZ Tell Any Trick To Remember The Negative Inductive Effect Order

Inductive Effect And Its Applications Organic Chemistry YouTube

Introduction To Organic Chemistry Part I Ppt Download
What Is Negative Inductive Effect - [desc-12]