What Is Oxidation Number Of An Atom Oxidation Definition Oxidation is the loss of electrons or increase in oxidation state of a molecule atom or ion in a chemical reaction The opposite process is called reduction
This page discusses the various definitions of oxidation and reduction redox in terms of the transfer of oxygen hydrogen and electrons It also explains the terms oxidizing The oxidation state also referred to as the amount of oxidation defines a chemical compound s degree of oxidation loss of electrons of an atom Antoine Lavoisier first used the term
What Is Oxidation Number Of An Atom

What Is Oxidation Number Of An Atom
https://i.ytimg.com/vi/r3nuk19ZS9c/maxresdefault.jpg
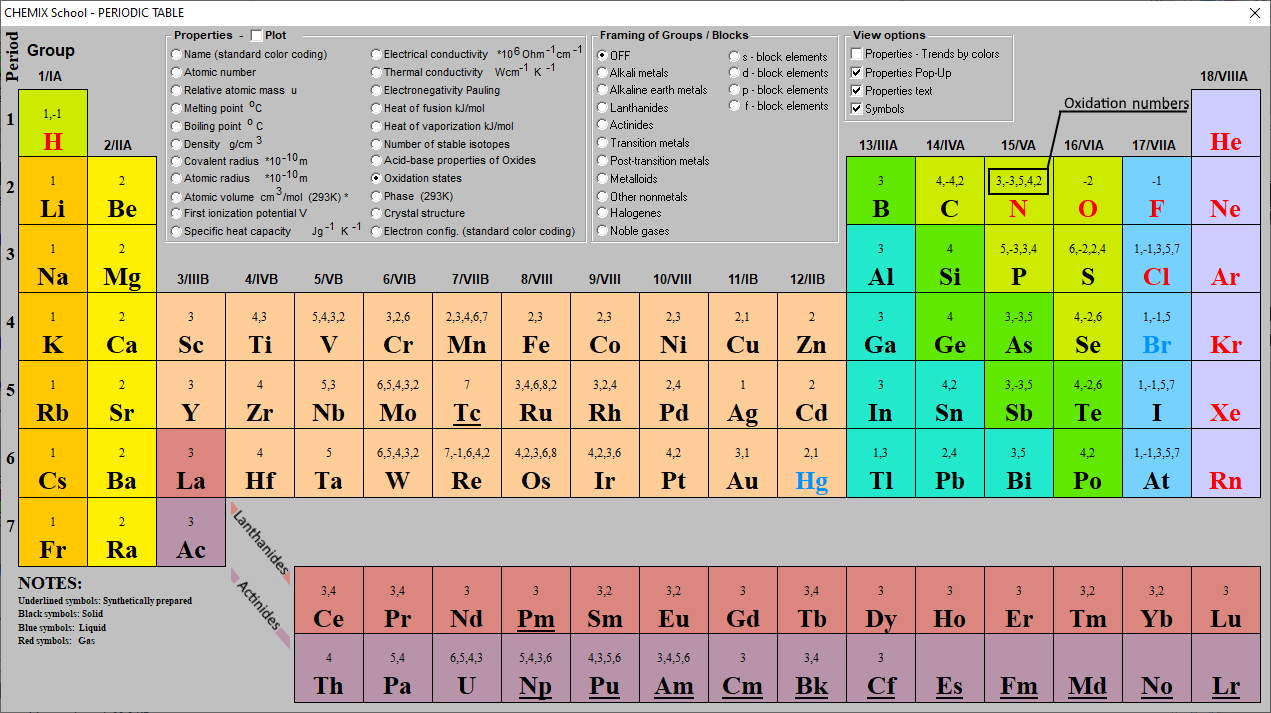
Oxidation Numbers Periodic Table Elements
https://www.chemix-chemistry-software.com/images/screenshots/periodic_table/version 8/oxidation-numbers.png

Oxidation State Examples Online Chemistry Tutor
https://www.onlinechemistrytutor.net/wp-content/uploads/2016/12/Chlorine.001-800x600.jpg
Oxidation When an atom or compound loses one or more electrons this is called oxidation Oxidation process In the oxidation process the properties of the atom or compound Oxidation is the process when an atom loses an electron in a reaction with oxygen and water A browned apple or a bicycle is a common phenomenon of oxidation
Oxidation is defined as a process in which an electron is removed from a molecule during a chemical reaction What happens in oxidation During oxidation there is a transfer of Oxidation is the loss of electrons of an atom ion or atoms in molecules during a chemical reaction Oxidation is an increase in the oxidation state of an atom Oxidation and
More picture related to What Is Oxidation Number Of An Atom
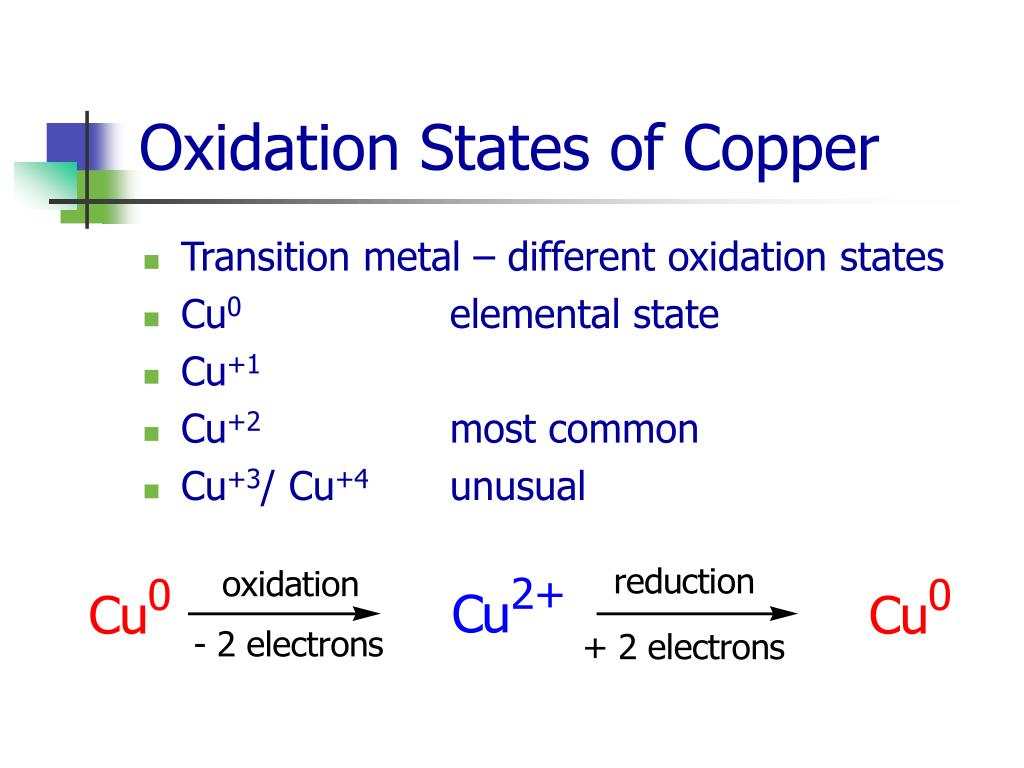
Cu 2 Cu0 Cu 2
https://image.slideserve.com/243127/oxidation-states-of-copper-l.jpg

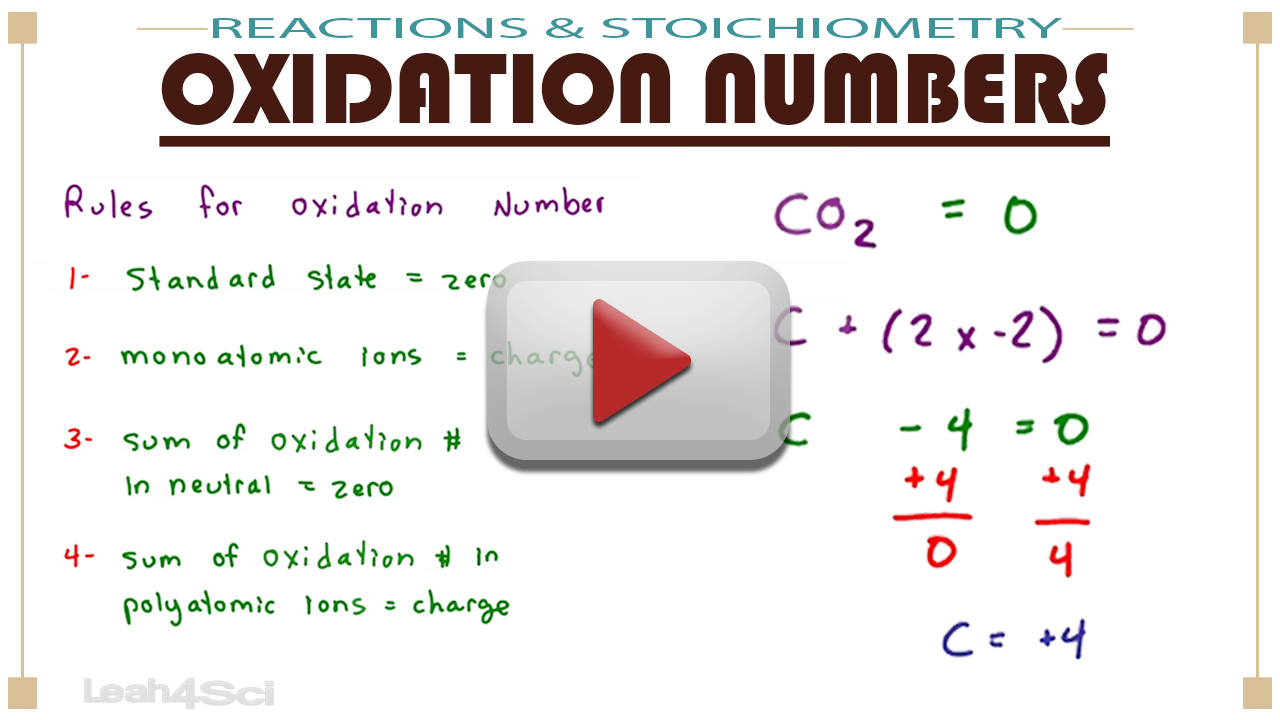
PPT Oxidation Numbers Rules PowerPoint Presentation Free Download
https://image3.slideserve.com/5491891/oxidation-numbers-rules-n.jpg
Oxidation Number Practice Worksheet Worksheets For Kindergarten
https://leah4sci.com/wp-content/uploads/2018/01/Calculating-Oxidation-Numbers-in-MCAT-General-Chemistry-8.1.jpg
Oxidation is when a substance reacts and combines with oxygen Combustion burning is an example of an oxidation reaction Oxidation is defined as the interaction between oxygen molecules and all the different substances they may contact from metal to living tissue Technically however with
[desc-10] [desc-11]
Rekacompanion Blog
https://s3.studylib.net/store/data/007610548_2-cfe9ca97347fa08110828e529eaacf6d.png
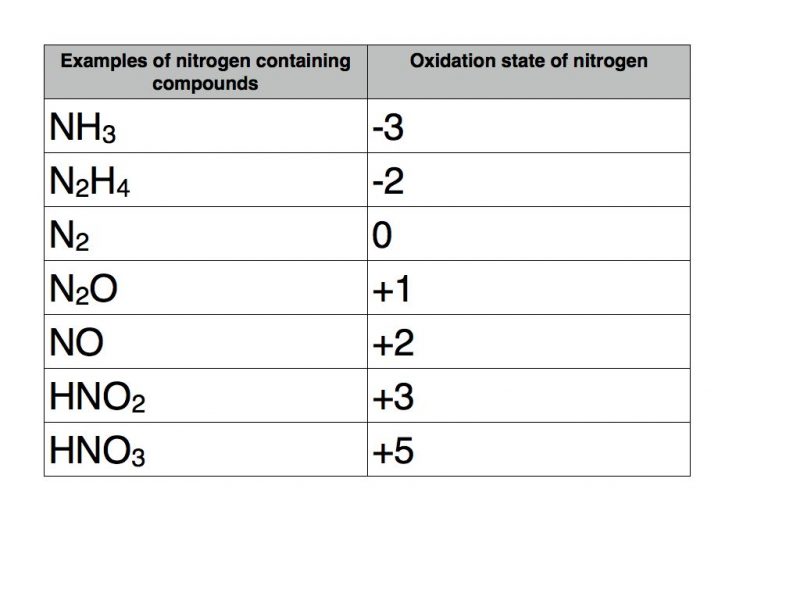
Oxidation Number
https://www.onlinechemistrytutor.net/wp-content/uploads/2016/12/Nitrogen.001-800x600.jpg

https://sciencenotes.org › what-is-oxidation-definition-and-examples
Oxidation Definition Oxidation is the loss of electrons or increase in oxidation state of a molecule atom or ion in a chemical reaction The opposite process is called reduction

https://chem.libretexts.org › Bookshelves › Analytical...
This page discusses the various definitions of oxidation and reduction redox in terms of the transfer of oxygen hydrogen and electrons It also explains the terms oxidizing

Oxidation Numbers On The Periodic Table Diagram Quizlet

Rekacompanion Blog

Oxidation Number State Definition Rules How To Find And Examples

Oxidation Number State Definition Rules How To Find And Examples
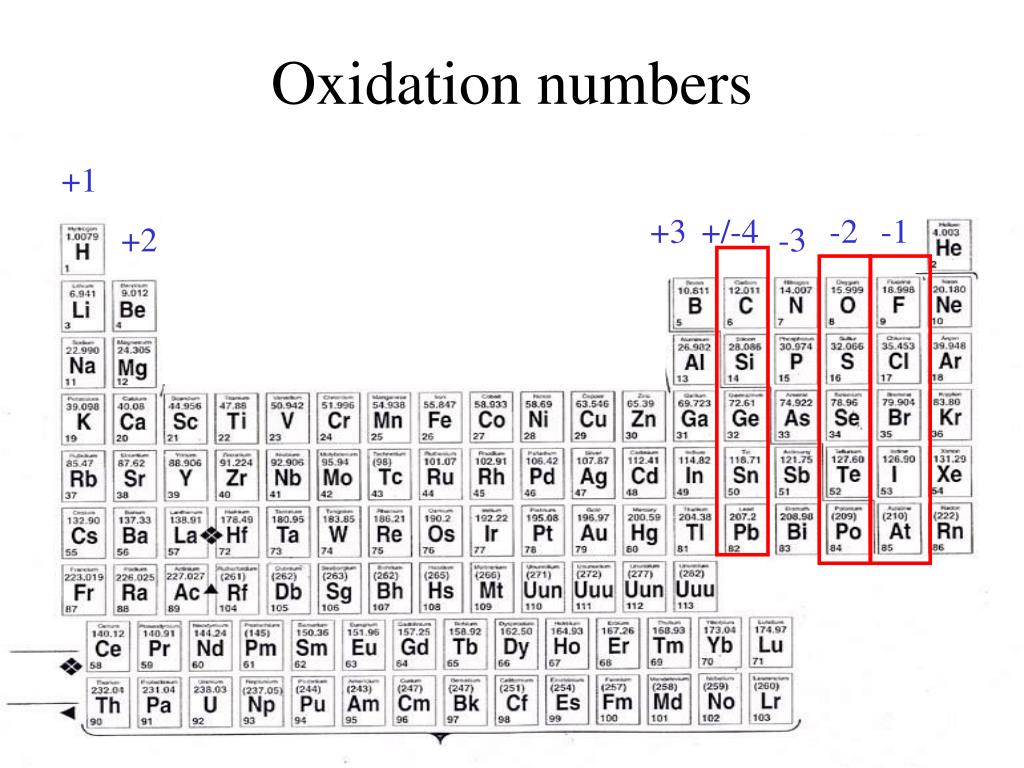
Oxidation Chart Periodic Table Loperstest

Question Video Deducing The Oxidation State Of Oxygen In Hydrogen

Question Video Deducing The Oxidation State Of Oxygen In Hydrogen
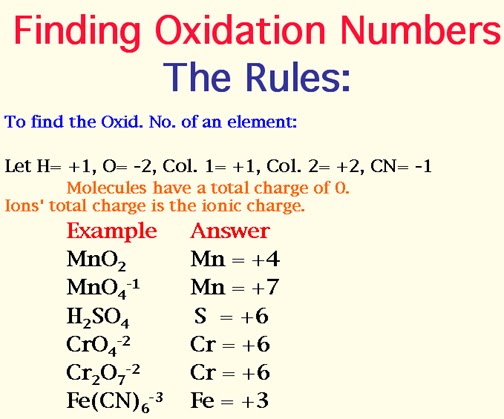
Chemistry Redox Reaction Rules For Assigning Oxidation Number
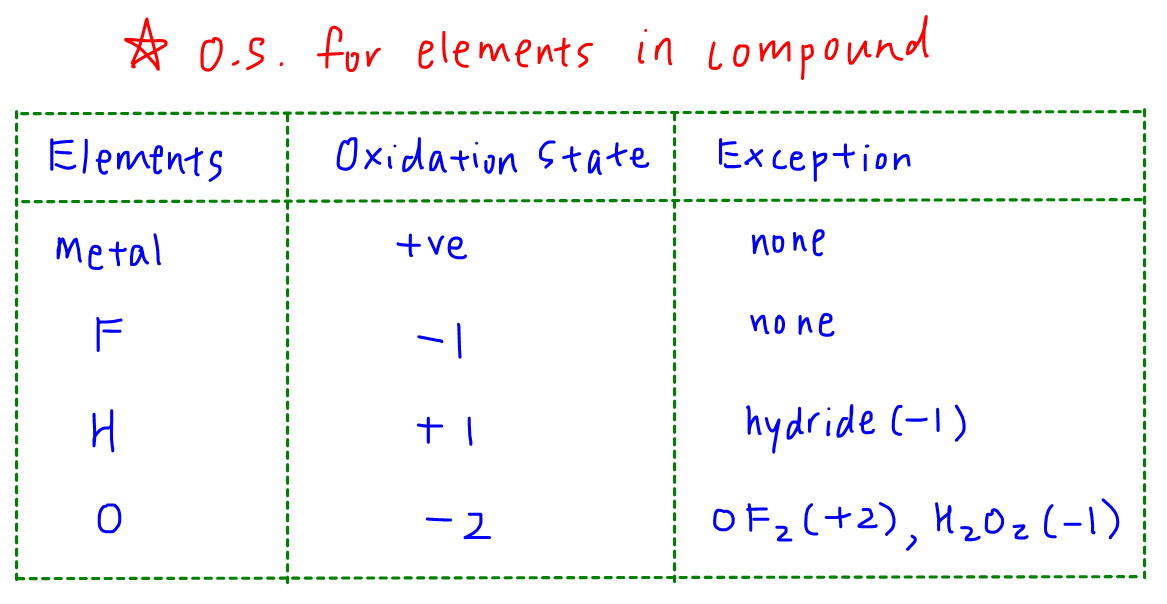
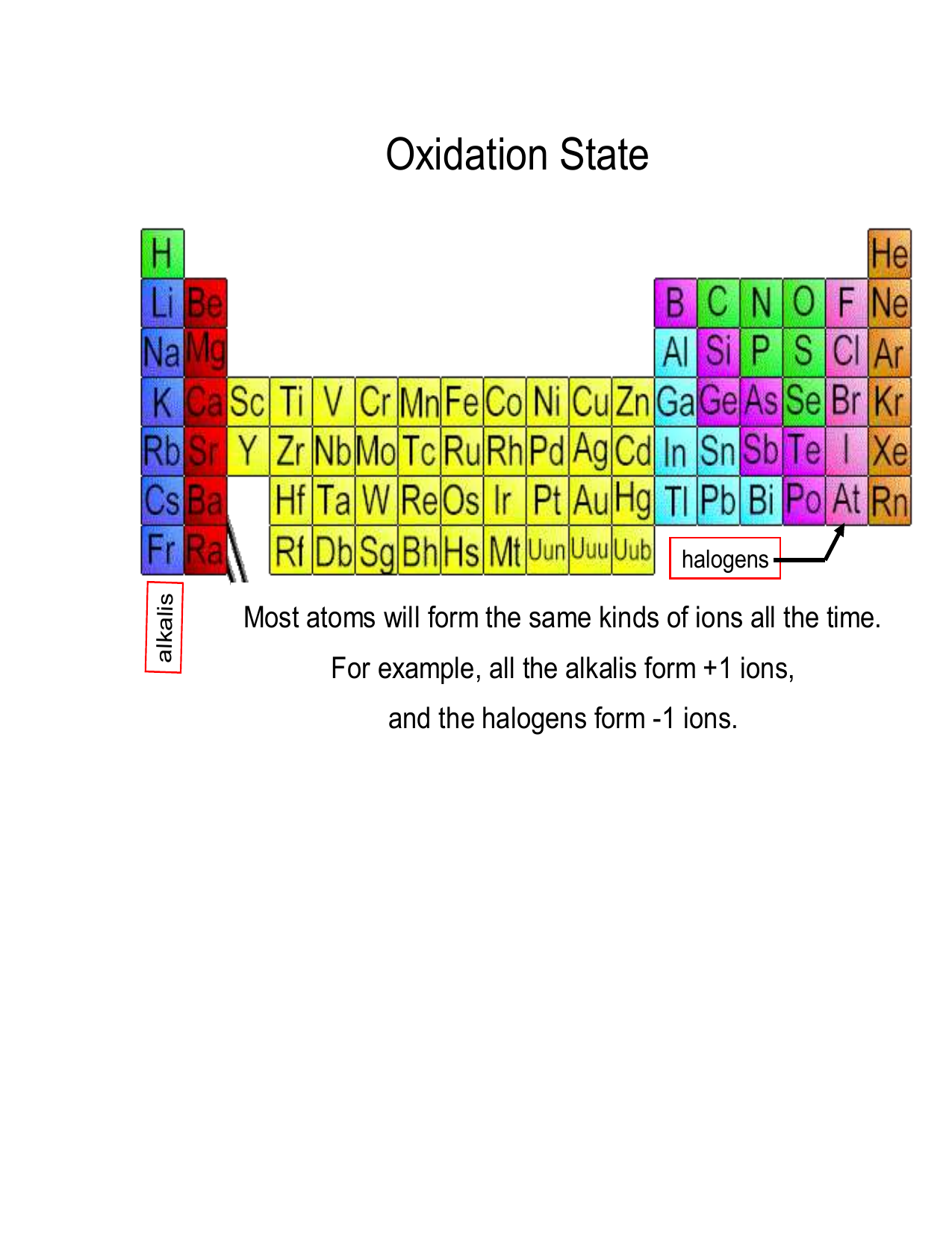
Oxidation State

Oxidationszahlen Tabelle
What Is Oxidation Number Of An Atom - Oxidation When an atom or compound loses one or more electrons this is called oxidation Oxidation process In the oxidation process the properties of the atom or compound