What Is The Oxidation State Of The Manganese Atom In The Permanganate Ion Oxidation is the loss of electrons during a reaction by a molecule atom or ion Oxidation occurs when the oxidation state of a molecule atom or ion is increased
This page discusses the various definitions of oxidation and reduction redox in terms of the transfer of oxygen hydrogen and electrons It also explains the terms oxidizing agent and Oxidation is caused when an atom a molecule or even an ion comes in contact with oxygen When this happens it transfers electrons and changes to get a more stable
What Is The Oxidation State Of The Manganese Atom In The Permanganate Ion

What Is The Oxidation State Of The Manganese Atom In The Permanganate Ion
https://i.ytimg.com/vi/pUGMjbd9Mio/maxresdefault.jpg

How To Find The Oxidation Number For Mn In The MnO2 2 Ion Manganate
https://i.ytimg.com/vi/g4gRCXDNnDo/maxresdefault.jpg

The Diagram Shows An Arrangement Of Different Types Of Reactions In
https://i.pinimg.com/originals/62/0e/ef/620eefa9d372f7bdf151c9f3ac7f5d44.png
Oxidation is the process when an atom loses an electron in a reaction with oxygen and water A browned apple or a bicycle is a common phenomenon of oxidation Oxidation is an increase in the oxidation state of an atom Oxidation and reduction form a redox reaction that is remembered by the acronym OIL RIG Oxidation Is Loss
In an oxidation reaction a substance gains oxygen atoms Learn more in this KS3 Chemistry guide from Bitesize Oxidation is defined as the interaction between oxygen molecules and all the different substances they may contact from metal to living tissue Technically however with
More picture related to What Is The Oxidation State Of The Manganese Atom In The Permanganate Ion

Manganese Compounds Colors different Oxidation States chemistry
https://i.pinimg.com/736x/63/a0/94/63a0948d3b604999020fea1dae9989bd.jpg
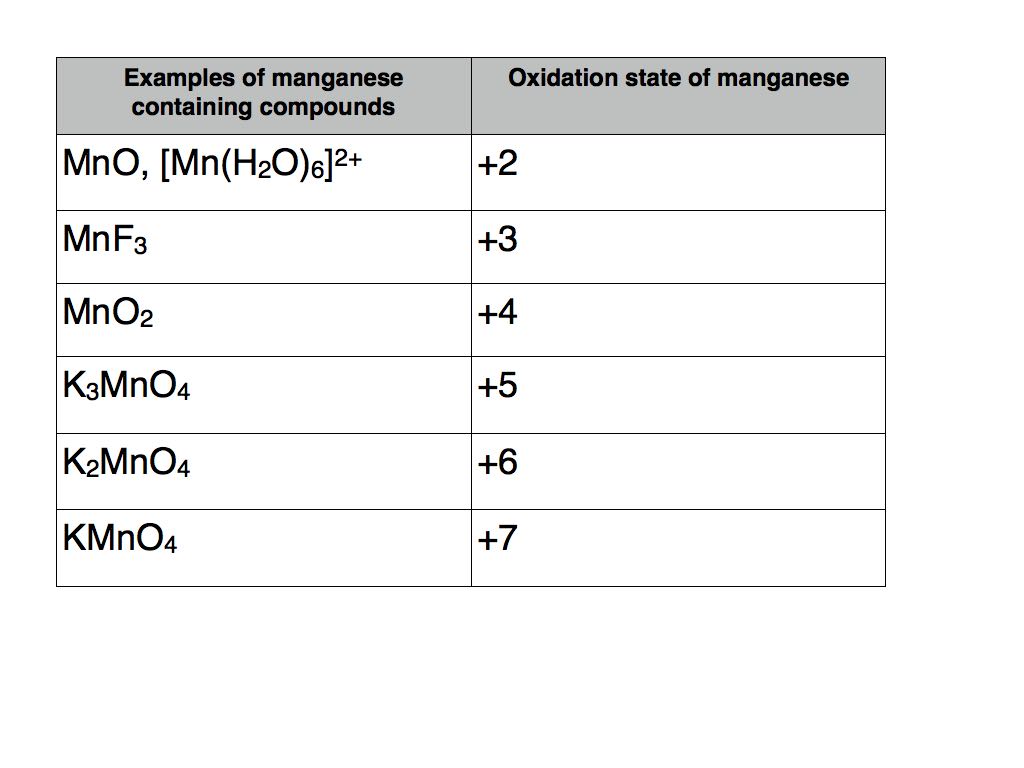
Oxidation State Examples Online Chemistry Tutor
https://www.onlinechemistrytutor.net/wp-content/uploads/2016/12/manganese.001.jpg

Draw The Structure Of Manganate On And Permanganate Ion Brainly in
https://hi-static.z-dn.net/files/da7/606afb277e64670053470743f5de971c.jpg
Oxidation is a chemical reaction that involves the loss of electrons by an atom ion or molecule It is a fundamental concept in the study of redox reduction oxidation reactions which are Oxidation involves the loss of electrons during a reaction by a molecule atom or ion This process is vital in various reactions such as combustion rusting and even cellular
[desc-10] [desc-11]

Question Video Identifying The Correct Sequence Of Orbital Diagrams To
https://media.nagwa.com/840104164268/en/thumbnail_l.jpeg

India Mineral Map Mineral Resources In India UPSC
https://lotusarise.com/wp-content/uploads/2024/03/Manganese-Ore-Distribution-in-India-1765x2048.png

https://www.thoughtco.com
Oxidation is the loss of electrons during a reaction by a molecule atom or ion Oxidation occurs when the oxidation state of a molecule atom or ion is increased

https://chem.libretexts.org › Bookshelves › Analytical...
This page discusses the various definitions of oxidation and reduction redox in terms of the transfer of oxygen hydrogen and electrons It also explains the terms oxidizing agent and
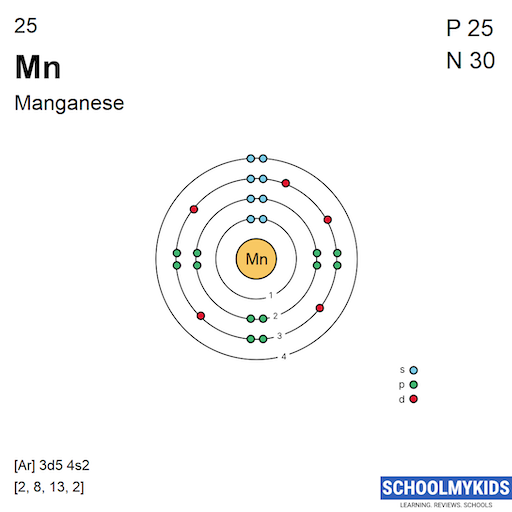
Manganese Bohr Model

Question Video Identifying The Correct Sequence Of Orbital Diagrams To

Manganese Bohr Model
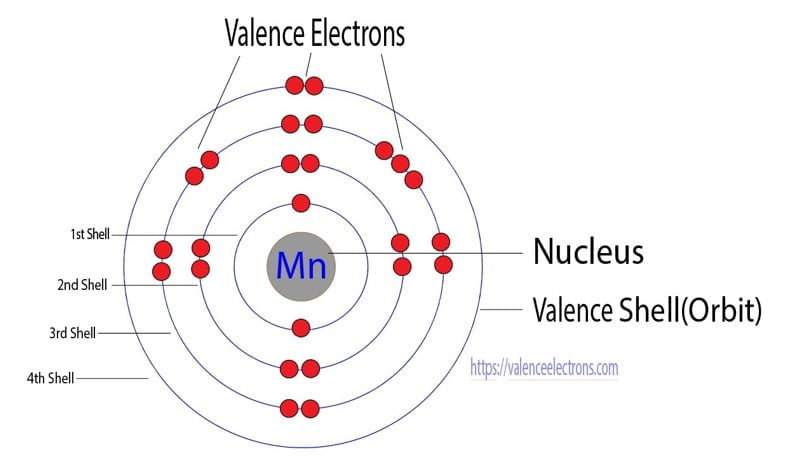
Manganese Atom Model
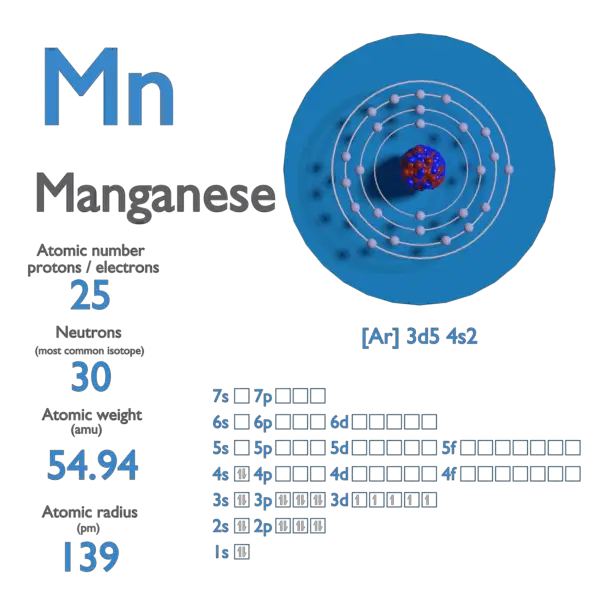
Manganese Atom Model
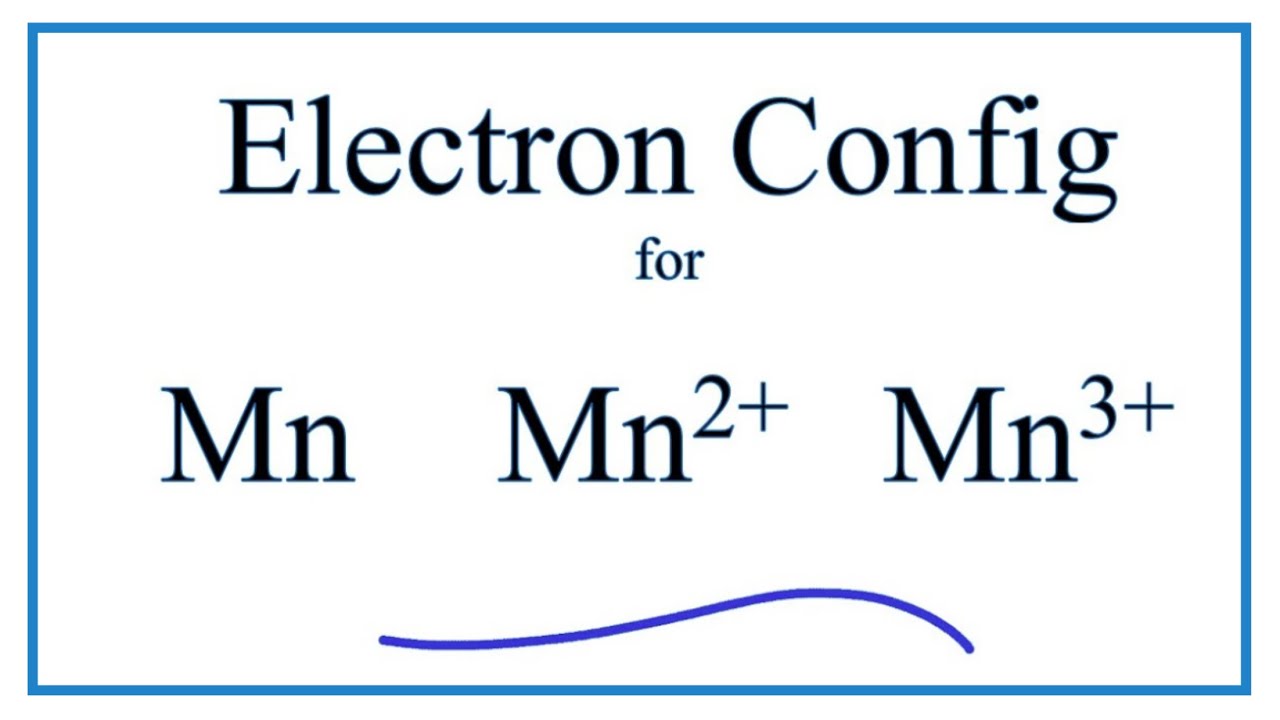
Manganese Atom Model

Manganese Atom Model

Manganese Atom Model

Manganese Atom Model
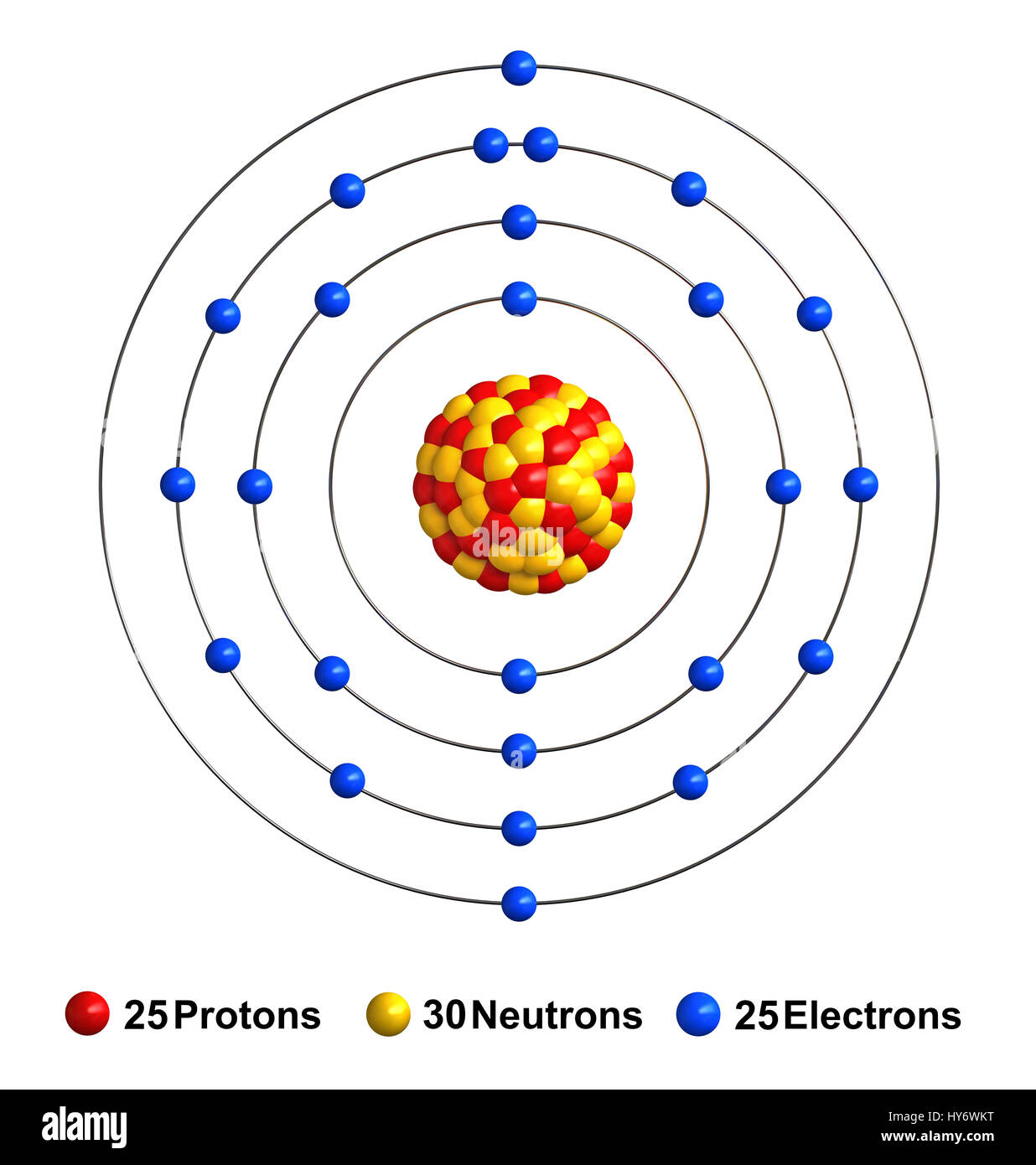
Manganese Atom Model
What Is The Oxidation State Of The Manganese Atom In The Permanganate Ion - Oxidation is an increase in the oxidation state of an atom Oxidation and reduction form a redox reaction that is remembered by the acronym OIL RIG Oxidation Is Loss