What Is The Ph Value Of Pure Water Answer PH quantitative measure of the acidity or basicity of aqueous or other liquid solutions The term widely used in chemistry biology and agronomy translates the values of
This article explains the concept of pH how to find and calculate the pH and how the pH formula and pH equation are used in chemistry PH is a measure of how acidic or basic a solution is from 0 to 14 pH is important for chemical reactions in areas like medicine cooking and agriculture Scientists use pH
What Is The Ph Value Of Pure Water Answer

What Is The Ph Value Of Pure Water Answer
http://i1.ytimg.com/vi/1uKtNlPLPZw/maxresdefault.jpg
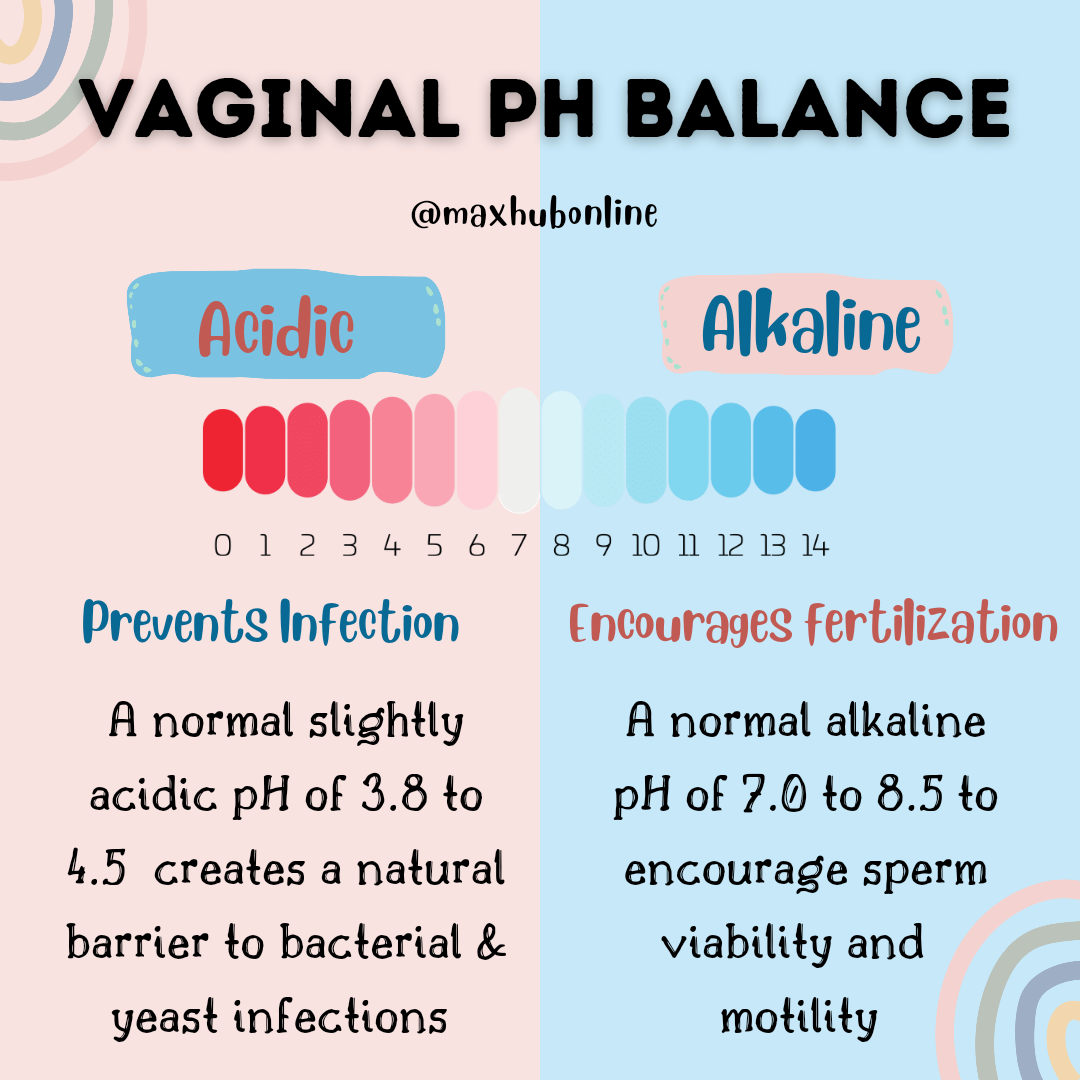
Health Posts Maxhub Pharmacy
https://maxhubpharm.com/wp-content/uploads/2022/11/20221116_094018_0000.png

PH And POH Senior Chemistry Saints
http://saintssnrchem.weebly.com/uploads/3/8/1/9/38195043/1439065542.png
The pH level or possible level of hydrogen in your body is determined by the food and type of drink you consume The pH is the concentration of the hydrogen ions This calculation is The term pH stands for potential of hydrogen and it is a unit of measurement for the concentration of hydrogen ions in a solution The concept of pH was first introduced by
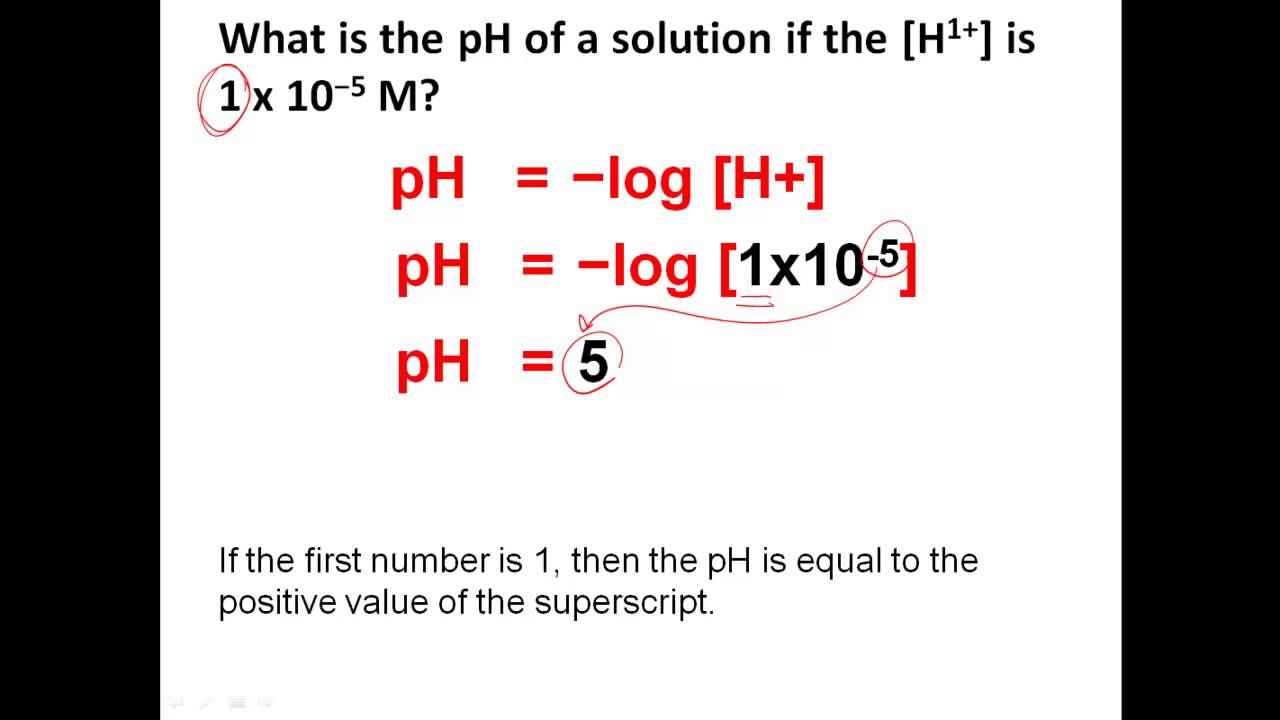
To calculate pH take the log of the hydrogen ion concentration and change the sign of the answer In chemistry pH is a number that acidity or basicity alkalinity of an PH is defined as the negative log of hydrogen ion concentration It can be used to describe the relative acidity or basicity of a solution Because it is based on a logarithmic scale a change
More picture related to What Is The Ph Value Of Pure Water Answer

What Is PH Definition Overview Expii EroFound
https://d20khd7ddkh5ls.cloudfront.net/what_is_ph_scale.png

What Is The PH Value Of Pure Water YouTube
https://i.ytimg.com/vi/zhdCIY9-Dr4/maxresdefault.jpg
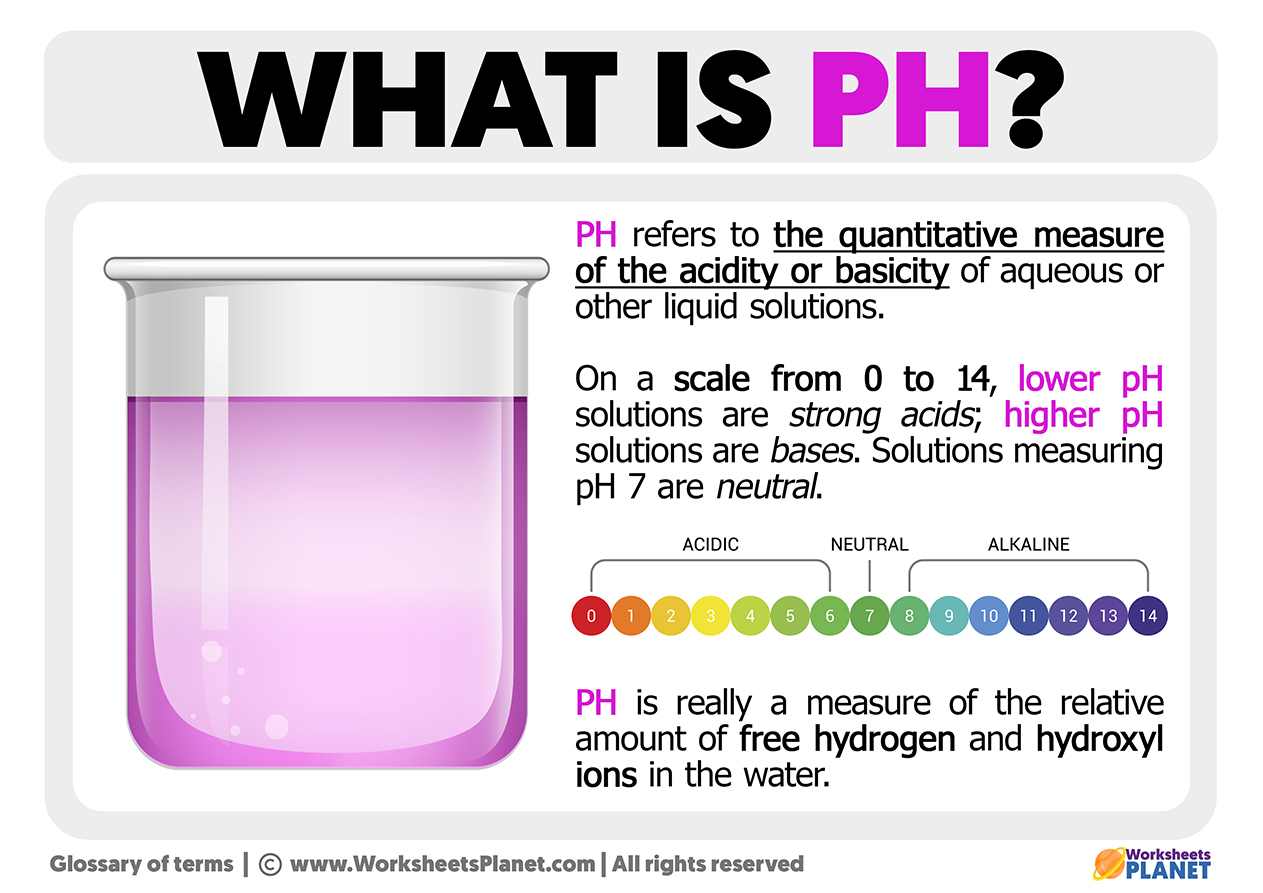
What Is PH
https://www.worksheetsplanet.com/wp-content/uploads/2022/12/What-is-PH.jpg
Most substances have a pH in the range of 0 to 14 although extremely acidic or alkaline substances may have pH 0 or pH 14 Alkaline substances have instead of hydrogen ions PH measures if a solution is acidic or alkaline with a scale from 0 to 14 The pH equation is pH log H where H is the hydrogen ion concentration in moles per liter pH
[desc-10] [desc-11]

PH Scale Definition Chart Values Range
https://www.chemistrylearner.com/wp-content/uploads/2023/03/pH-Scale-768x402.jpg
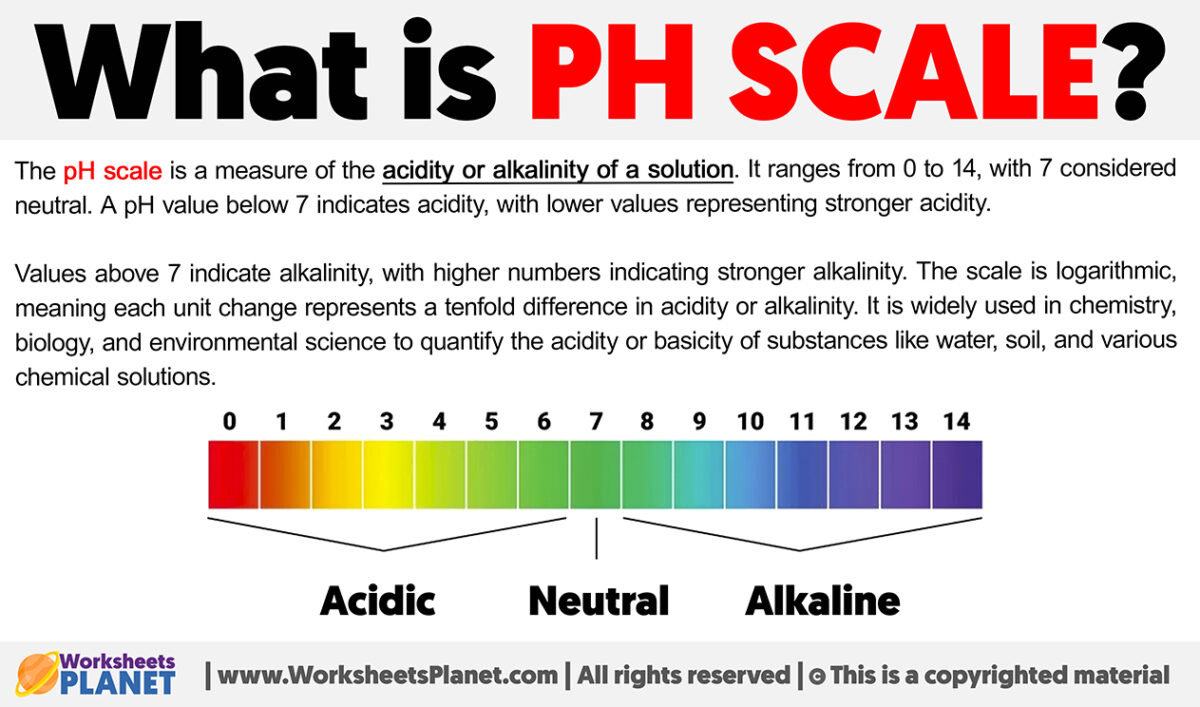
What Is Ph Scale Definition Of Ph Scale
https://www.worksheetsplanet.com/wp-content/uploads/2024/01/What-is-Ph-Scale-1200x707.jpg
https://www.britannica.com › science › pH
PH quantitative measure of the acidity or basicity of aqueous or other liquid solutions The term widely used in chemistry biology and agronomy translates the values of

https://chemistrytalk.org › what-is-ph
This article explains the concept of pH how to find and calculate the pH and how the pH formula and pH equation are used in chemistry
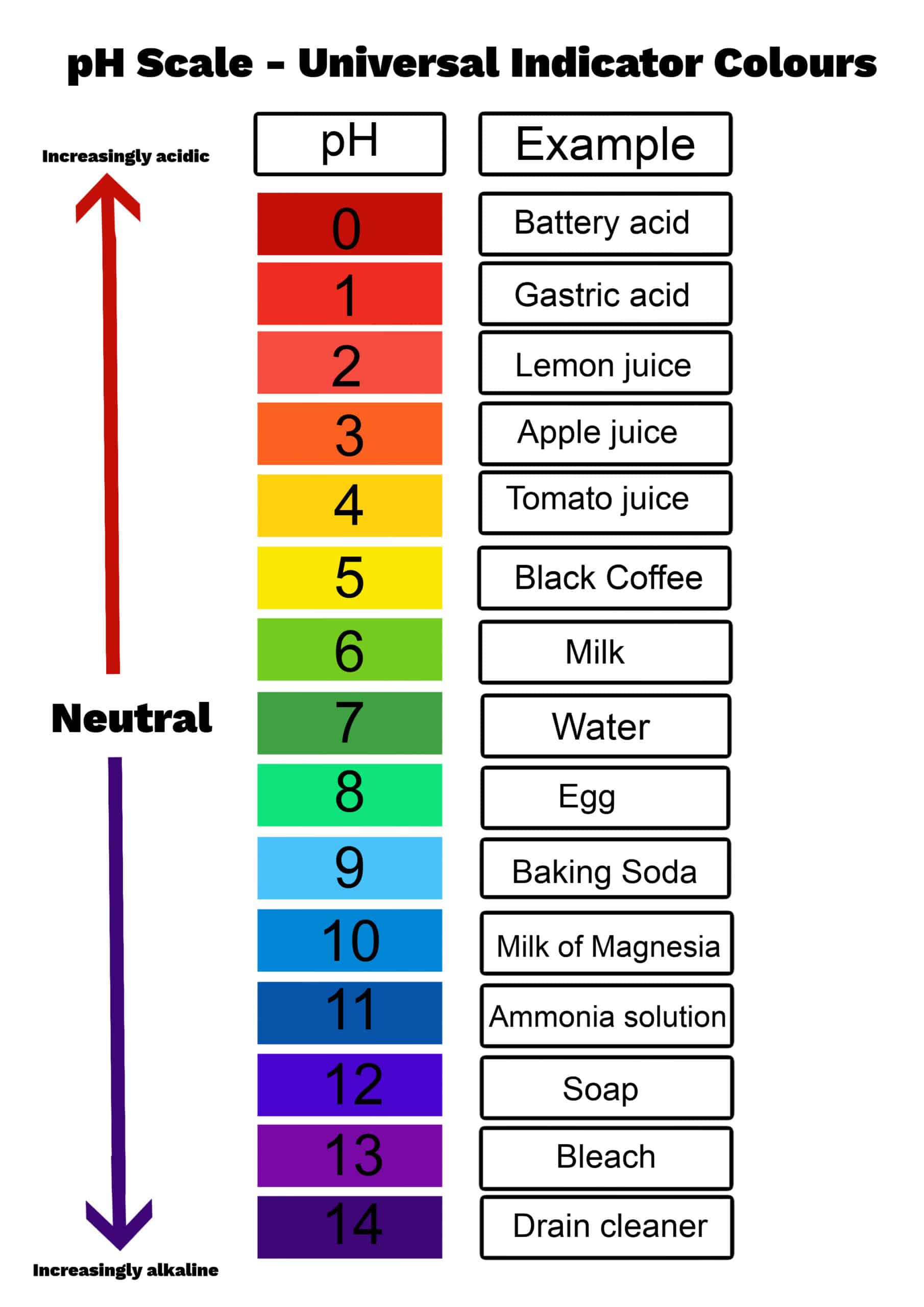
What Is The PH Scale

PH Scale Definition Chart Values Range

Ph Scale Examples

Https www bing search q francis portugal form QBLH sp 1 pq

Hydrangea Color Chart Navigate Hues For The Perfect Garden Palette
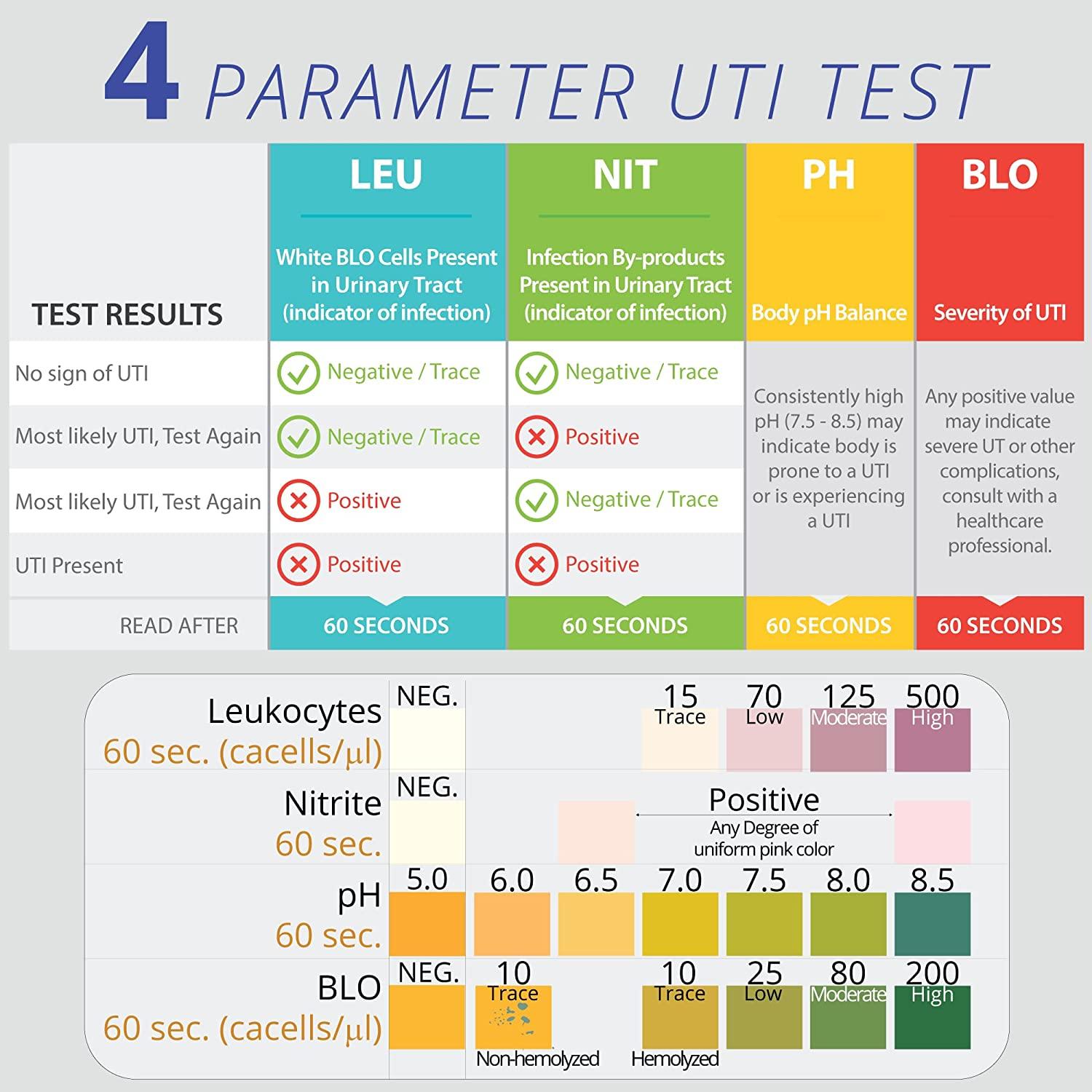
Urine PH Normal PH Levels Range Chart Causes Of 60 OFF

Urine PH Normal PH Levels Range Chart Causes Of 60 OFF
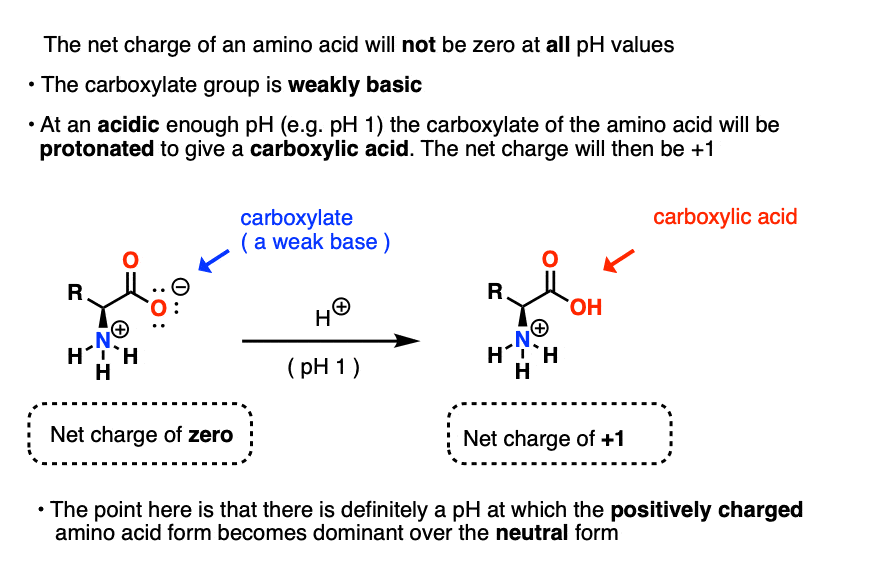
Formula For Determining Ph

Printable Ph Color Chart

Acids And Bases 8 3 And 8 4 Notes Ppt Download
What Is The Ph Value Of Pure Water Answer - PH is defined as the negative log of hydrogen ion concentration It can be used to describe the relative acidity or basicity of a solution Because it is based on a logarithmic scale a change