What Is Thermal Equilibrium Two objects are defined to be in thermal equilibrium if when placed in thermal contact no net energy flows from one to the other and their temperatures don t change We
Thermal Equilibrium An important concept related to temperature is thermal equilibrium Two objects are in thermal equilibrium if they are in close contact that allows either to gain energy The zeroth law of thermodynamics uses thermal equilibrium to define how two different systems can be said to be at the same temperature For example when molten rock comes up from a
What Is Thermal Equilibrium
What Is Thermal Equilibrium
https://thermal-engineering.org/wp-content/uploads/2019/05/Thermal-equilibrium-Zeroth-Law-Thermodynamics.png

What Is Thermal Equilibrium
https://i.ytimg.com/vi/NxUH-GDKS0M/maxresdefault.jpg

What Is Thermal Equilibrium Definition
https://i.ytimg.com/vi/FSfgxwYJsF4/maxresdefault.jpg
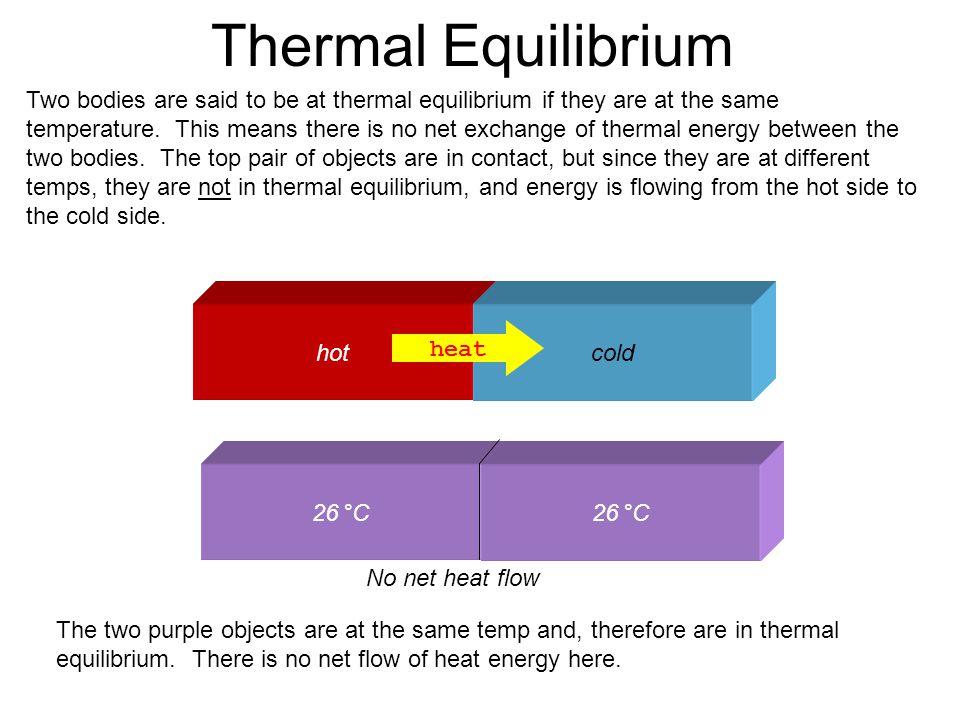
Defining thermal equilibrium Regions in thermal equilibrium have the same temperature Thermal equilibrium occurs when there is no longer thermal energy transfer When two substances having different temperatures are introduced or kept together heat energy flows from a substance at higher temperature to a substance at lower temperature Also heat
Thermal Equilibrium is a condition when two systems have the same temperature and there no more net flow of thermal energy It also obeys the zeroth law of thermodynamics Thermal equilibrium is a state in which two or more bodies or systems reach the same temperature and therefore no longer exchange thermal energy between them To
More picture related to What Is Thermal Equilibrium
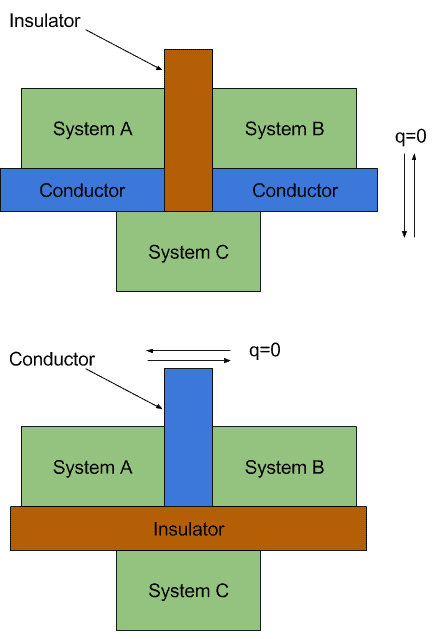
What Is Thermal Equilibrium In Chemistry
https://1.bp.blogspot.com/-d6WiBEy_zSA/V1lBixRl_8I/AAAAAAAAAHw/be1qE7mtdhoP4FdBzd_OBp8NoDkZFcuogCLcB/s1600/slide_20.jpg

What Is Thermal Equilibrium In Chemistry
https://i.ytimg.com/vi/7jAPitc0bkY/maxresdefault.jpg

What Is Thermal Equilibrium In Chemistry
https://i.ytimg.com/vi/TjTW6EjYHUE/maxresdefault.jpg
Thermal equilibrium is a state in which all parts of a system are at the same temperature This means that there is no net flow of thermal energy between different parts of the system When Thermal equilibrium is a concept used in the zeroth law of thermodynamics to explain how two dissimilar systems may be stated to be at the same temperature For instance when molten
[desc-10] [desc-11]

What Is Thermal Equilibrium Continuing To Innovate Thermal Imaging
https://i.ytimg.com/vi/LQVHQ0YL0R0/maxresdefault.jpg

What Is Thermal Equilibrium Kofarest PD Kofarest LS Kofarest LS
https://static-images.findfilo.com/classroom/1678014532472_nnqqcvbx_3569265.jpg

https://www.thermal-engineering.org › what-is...
Two objects are defined to be in thermal equilibrium if when placed in thermal contact no net energy flows from one to the other and their temperatures don t change We

https://phys.libretexts.org › Bookshelves › University...
Thermal Equilibrium An important concept related to temperature is thermal equilibrium Two objects are in thermal equilibrium if they are in close contact that allows either to gain energy

1 What Is Thermal Equilibrium 2 State The Characteristics Of Thermence

What Is Thermal Equilibrium Continuing To Innovate Thermal Imaging

Temperature Dependence Of Equilibrium Constant YouTube
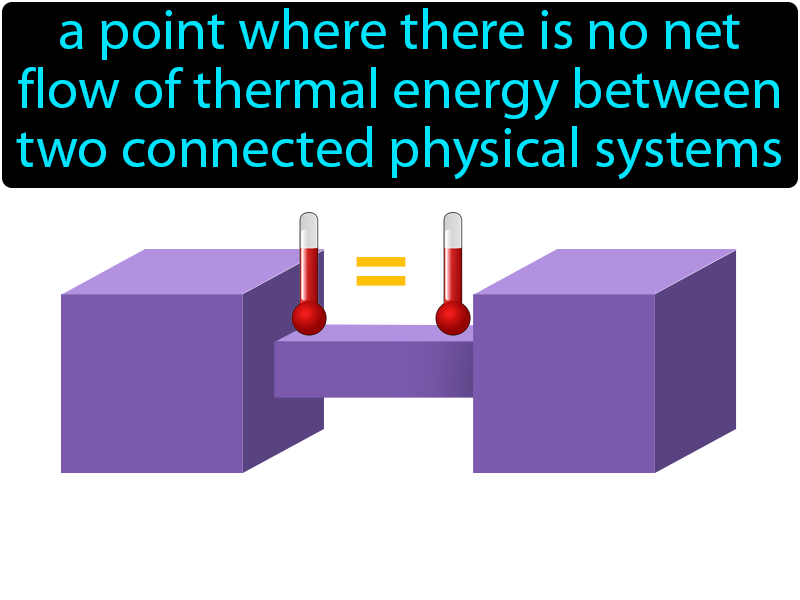
Thermal Equilibrium Definition Image Flippy Flashcards

Equilibrium Solutions To The Heat Equation YouTube

Equilibrium Thermal Chemical Mechanical Thermodynamic Basic

Equilibrium Thermal Chemical Mechanical Thermodynamic Basic

A Level Physics All Exam Boards Thermal Physics Thermal Equilibrium

Thermodynamics Equilibrium Zeroth Law Of Thermodynamics Steady
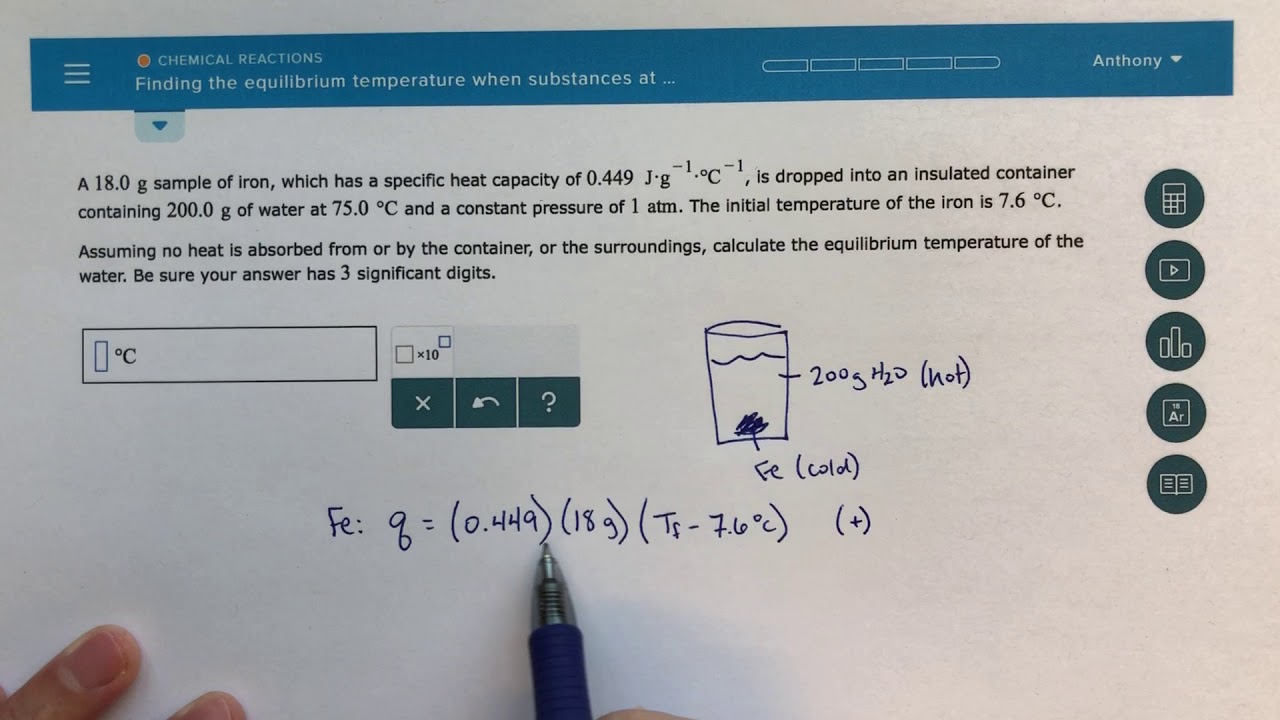
ALEKS Finding The Equilibrium Temperature When Substances At
What Is Thermal Equilibrium - [desc-13]