What Is Z Effective Chemistry The amount of positive charge experienced by any individual electron is the effective nuclear charge Z eff For example in lithium Li none of the
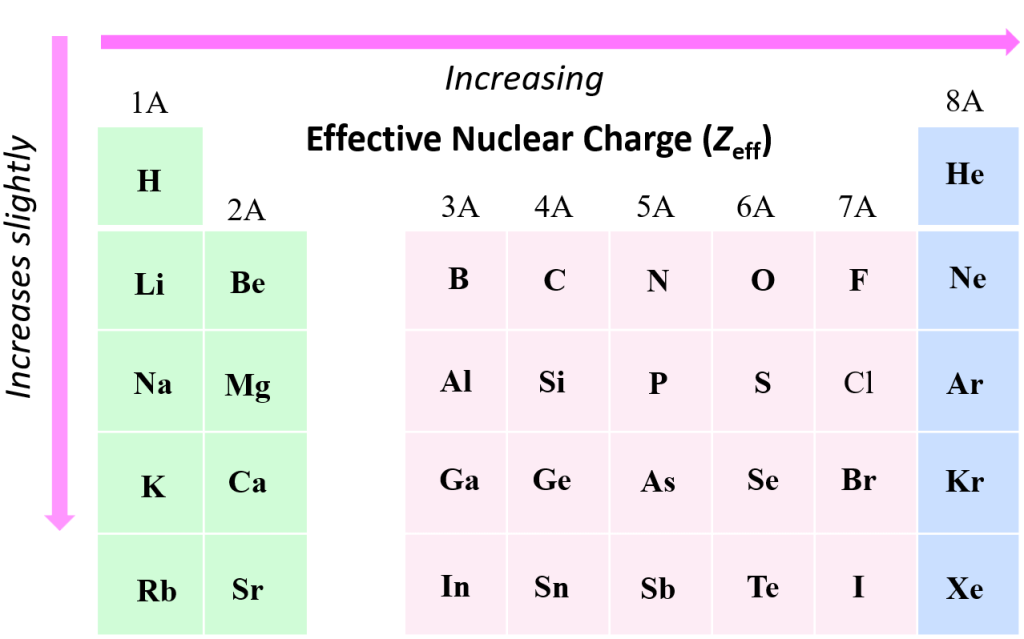
The trends in the valence Z are not simple because as atomic number increases the valence shell and or subshell also changes The valence Z is indicated in Figure Effective nuclear charge Z eff is the nuclear charge an electron actually experiences Let s understand what this statement means
What Is Z Effective Chemistry

What Is Z Effective Chemistry
https://image.isu.pub/220630071731-632a45e1c2097b2dda8ae5bd65ddbce7/jpg/page_1.jpg
Effective Nuclear Charge Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2023/11/Effective-NUclear-charge-and-periodic-table-1024x639.png

Effective Nuclear Charge Electronic Structure MCAT Content
https://i2.wp.com/cms.jackwestin.com/wp-content/uploads/2020/03/Zeff-effective-nuclear-charge-1.jpg?resize=684%2C398&ssl=1
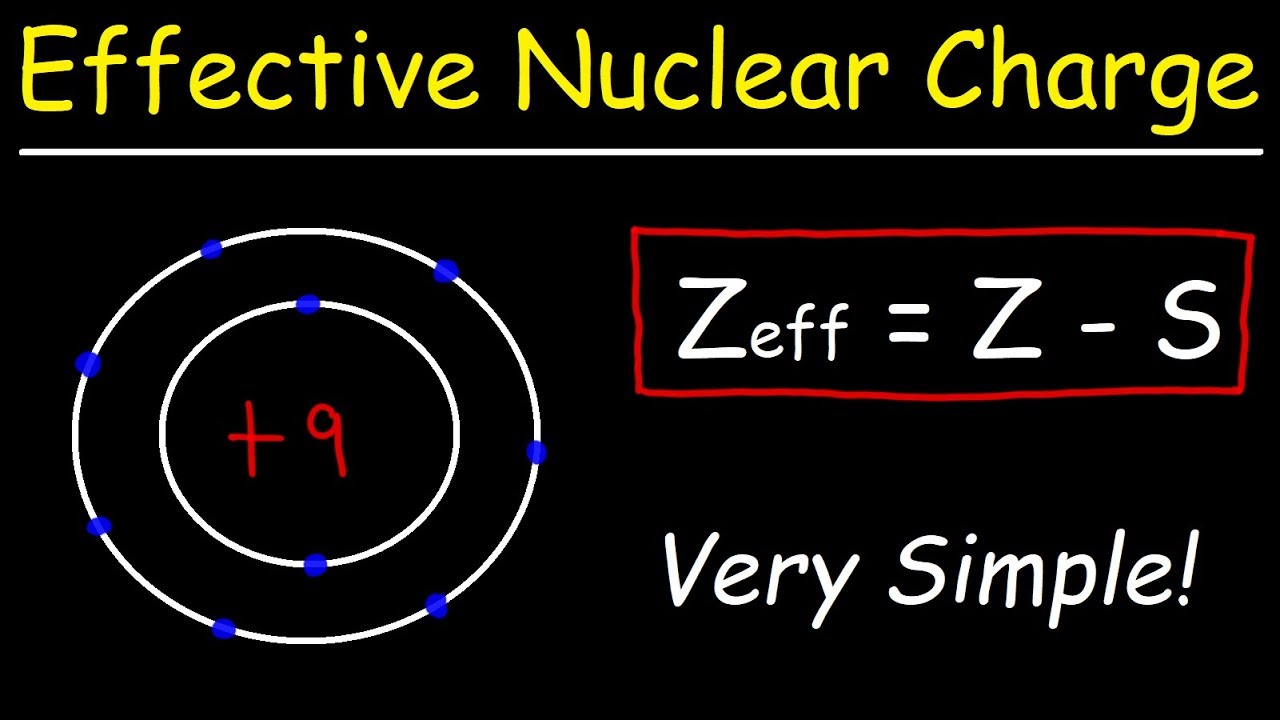
Electrons that are shielded from the full charge of the nucleus experience an effective nuclear charge Z eff of the nucleus which is some degree less than the full nuclear charge an electron would feel in a hydrogen atom or The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons The effective nuclear charge may be approximated by the equation
Here you will learn what the effective nuclear charge is and how to calculate it using Slater s rules You will see some examples and get a quick review of the quantum theory behind atoms and finally you will learn how Effective nuclear charge Z eff measures how strongly the nucleus pulls on a specific electron accounting for any electron electron repulsions For most atoms the inner electrons partially
More picture related to What Is Z Effective Chemistry

EFFECTIVE NUCLEAR CHARGE SLATER RULE Definition With Examples YouTube
https://i.ytimg.com/vi/buhRykOYwk8/maxresdefault.jpg

Z Effective Equation Importsokol
http://image.slidesharecdn.com/chapter8-141230113140-conversion-gate02/95/chapter-8-18-638.jpg?cb=1419960772
CHEMISTRY 101 Trends In Atomic Size And Effective Nuclear Charge YouTube
https://i.ytimg.com/vi/jBBDycqwyHk/maxresdefault.jpg
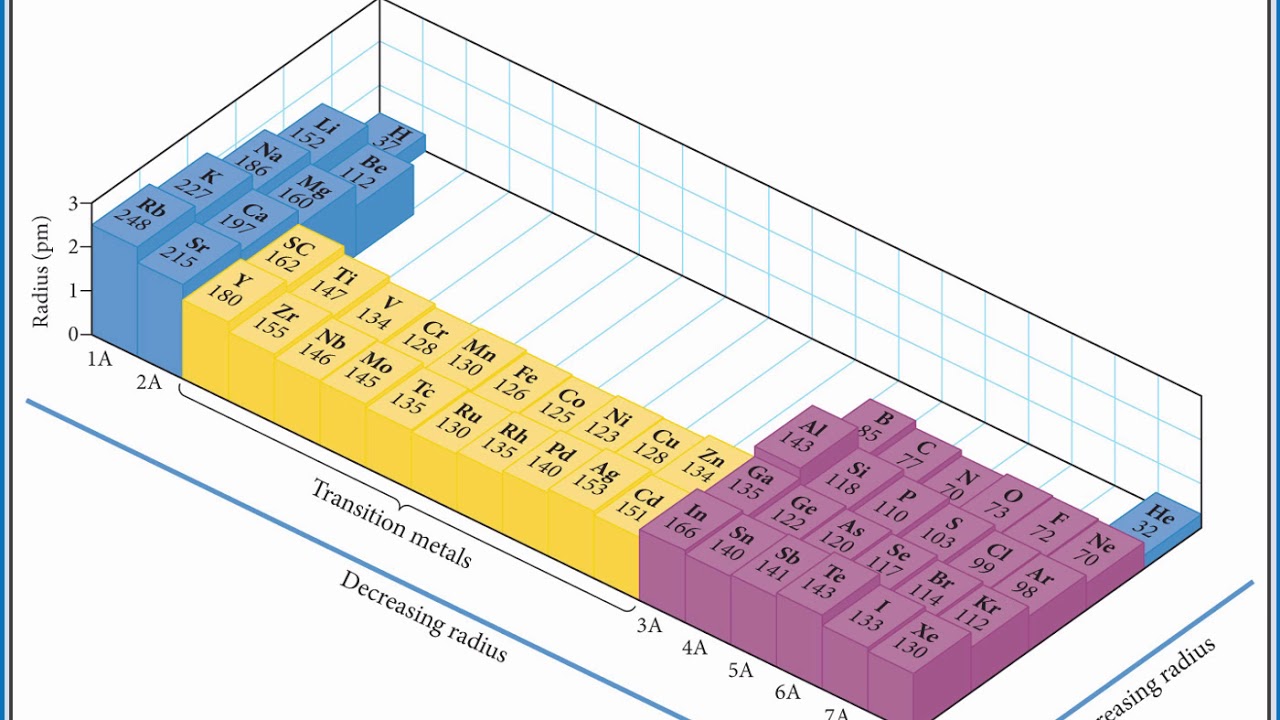
The concept of electron shielding in which intervening electrons act to reduce the positive nuclear charge experienced by an electron allows the use of hydrogen like orbitals and an effective nuclear charge Z eff to describe electron The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi electron atom The concept is used to help explain the electron configuration of atoms their sizes and their
Effective nuclear charge refers to the charge that the outermost valance electron have Also the electron or multi electron takes into account the number of shielding electrons that surrounds Z eff Effective nuclear charge Z Atomic number S Shielding constant The effective nuclear charge can be determined by using Slater s rule This rule calculates Z eff from the actual

Statistical Mechanics What Is Z In Collision Theory Of Chemical
https://i.stack.imgur.com/LKtWB.jpg
What Is Chemistry Definition Of Chemistry
https://www.worksheetsplanet.com/wp-content/uploads/2022/12/What-is-chemistry.jpg

https://chem.libretexts.org › Courses › Saint…
The amount of positive charge experienced by any individual electron is the effective nuclear charge Z eff For example in lithium Li none of the

https://chem.libretexts.org › Courses › Ursinus_College
The trends in the valence Z are not simple because as atomic number increases the valence shell and or subshell also changes The valence Z is indicated in Figure

How To Use Slater s Rule To Estimate The Effective Nuclear Charge YouTube

Statistical Mechanics What Is Z In Collision Theory Of Chemical

How To Find Nuclear Charge On Periodic Table Brokeasshome

Chemistry Background With Liquid Bottle Chemistry Formula Structure
How To Find Nuclear Charge On Periodic Table Brokeasshome

Some Basic Concept Of Chemistry Class 11 Formula PW 11th Chemistry

Some Basic Concept Of Chemistry Class 11 Formula PW 11th Chemistry

Buy A To Z CHEMISTRY NEET BOOKS OF BYJUS Book In Excellent Condition

Chemistry In Everyday Life Essay Exploring The Impact Of Chemistry In

Why Is Chemistry Called The Central Science
What Is Z Effective Chemistry - Here you will learn what the effective nuclear charge is and how to calculate it using Slater s rules You will see some examples and get a quick review of the quantum theory behind atoms and finally you will learn how