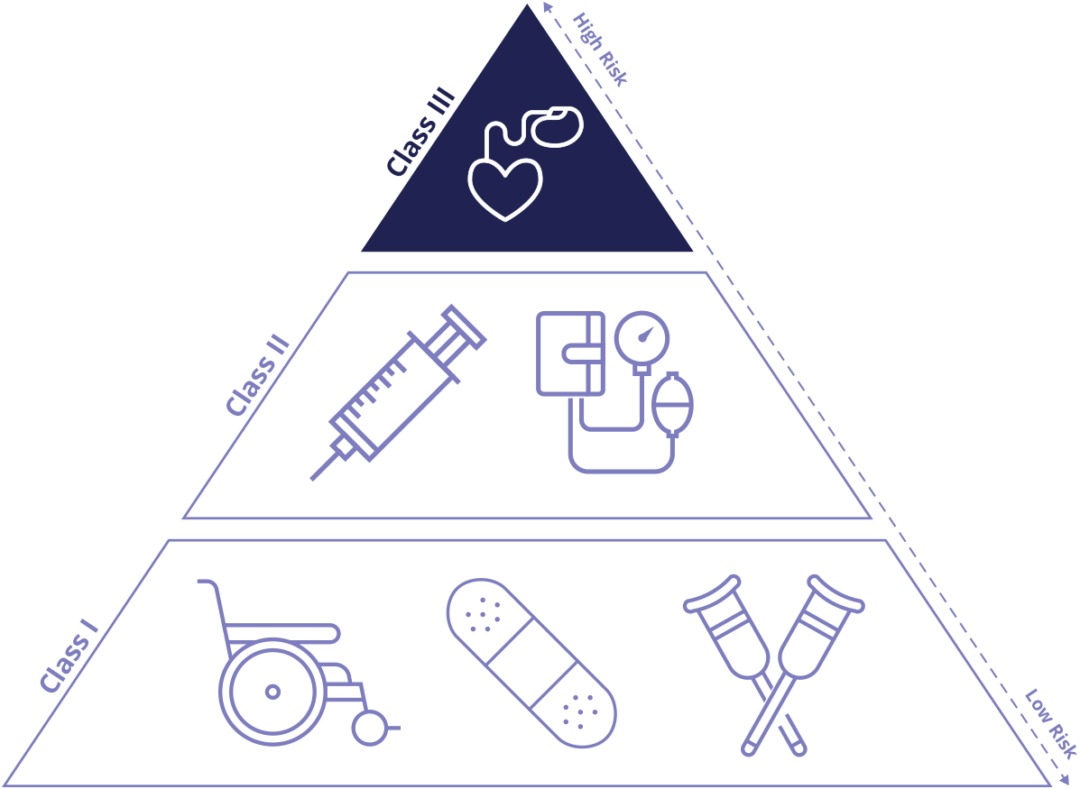
Class 1 Vs Class 2 Medical Device Class I includes devices with the lowest risk and Class III includes those with the greatest risk As indicated above all classes of devices as subject to General Controls
The Medical Devices Regulation sets requirements according to device class More specifically medical devices are defined as class I IIa IIb or III In turn this determines certain requirements and which conformity To get a basic understanding of what classes each regulatory organization has and what they mean let s take a brief look at each of them
Class 1 Vs Class 2 Medical Device

Class 1 Vs Class 2 Medical Device
https://i.ytimg.com/vi/BI-SxPJOsPQ/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGFAgWChlMA8=&rs=AOn4CLCvURb64pAL0CiOWGdCHkrnX-ZXfA

Angle s Classification Class II Division Introduction To 47 OFF
https://medinaz.com/blog/wp-content/uploads/2023/08/Class-II-Division-1-Vs-Class-II-Division-2.jpg
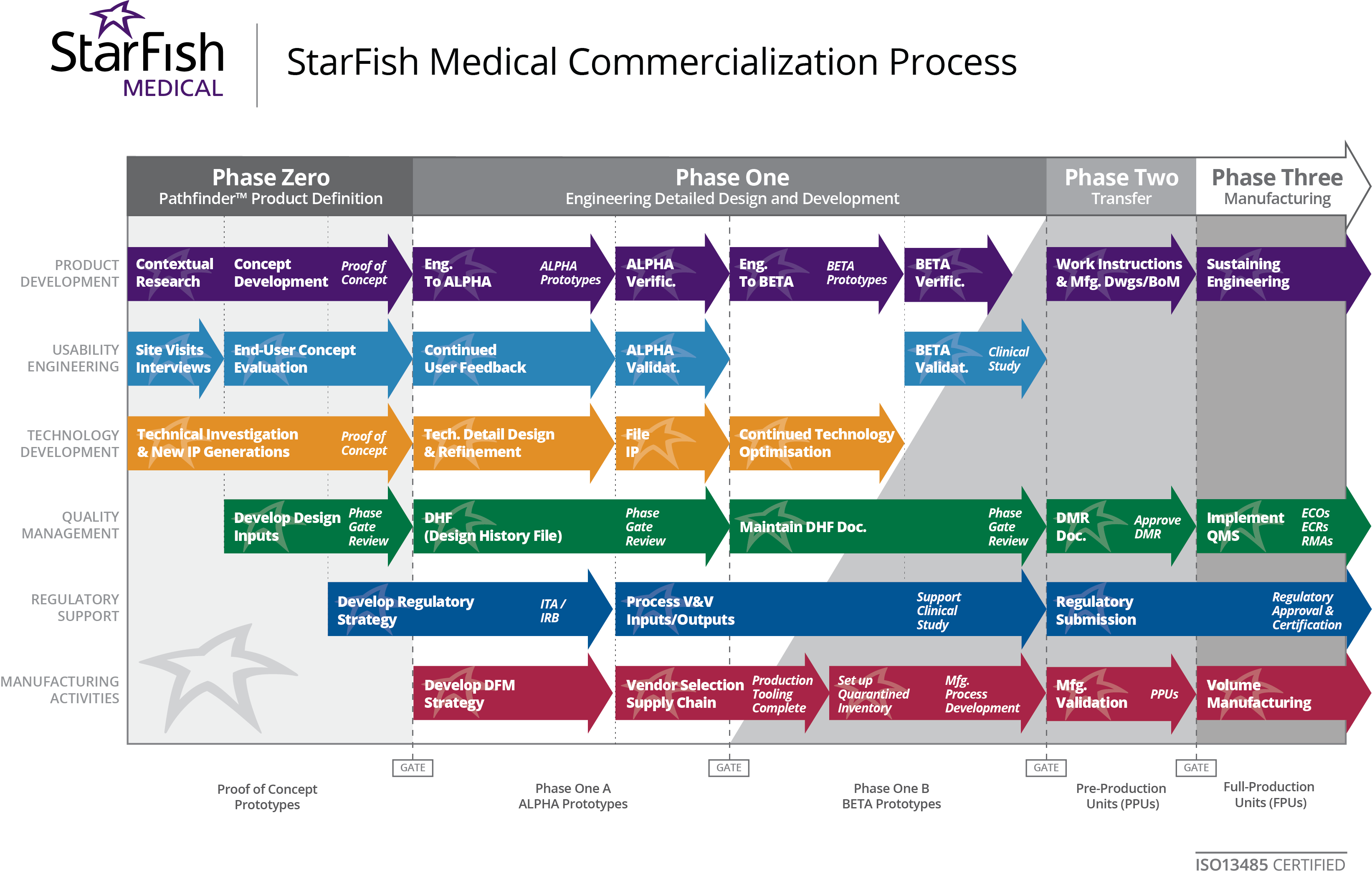
Medical Device Contract Manufacturing StarFish Medical
https://starfishmedical.com/assets/2021-Pathfinder-chart.png
The main difference between the class I medical device and class II medical device categories is the level of risk they pose to patients Class I devices are considered low risk and Class II devices are considered to be moderate risk Learn how the FDA classifies medical devices into three categories based on their risk and intended use Find out the differences between Class I II and III devices and see examples of each class
The Class I and Class II designations for electrical medical devices help ensure that people remain safe from electric shock with an appropriate level of protection Check the EN 60601 1 standard carefully when In the UK Medical Devices are categorised into 6 classes these are Class I Class Is sterile Class Im measuring Class IIa Class IIb and Class III Class I devices are considered to be of low risk Class II devices as medium
More picture related to Class 1 Vs Class 2 Medical Device
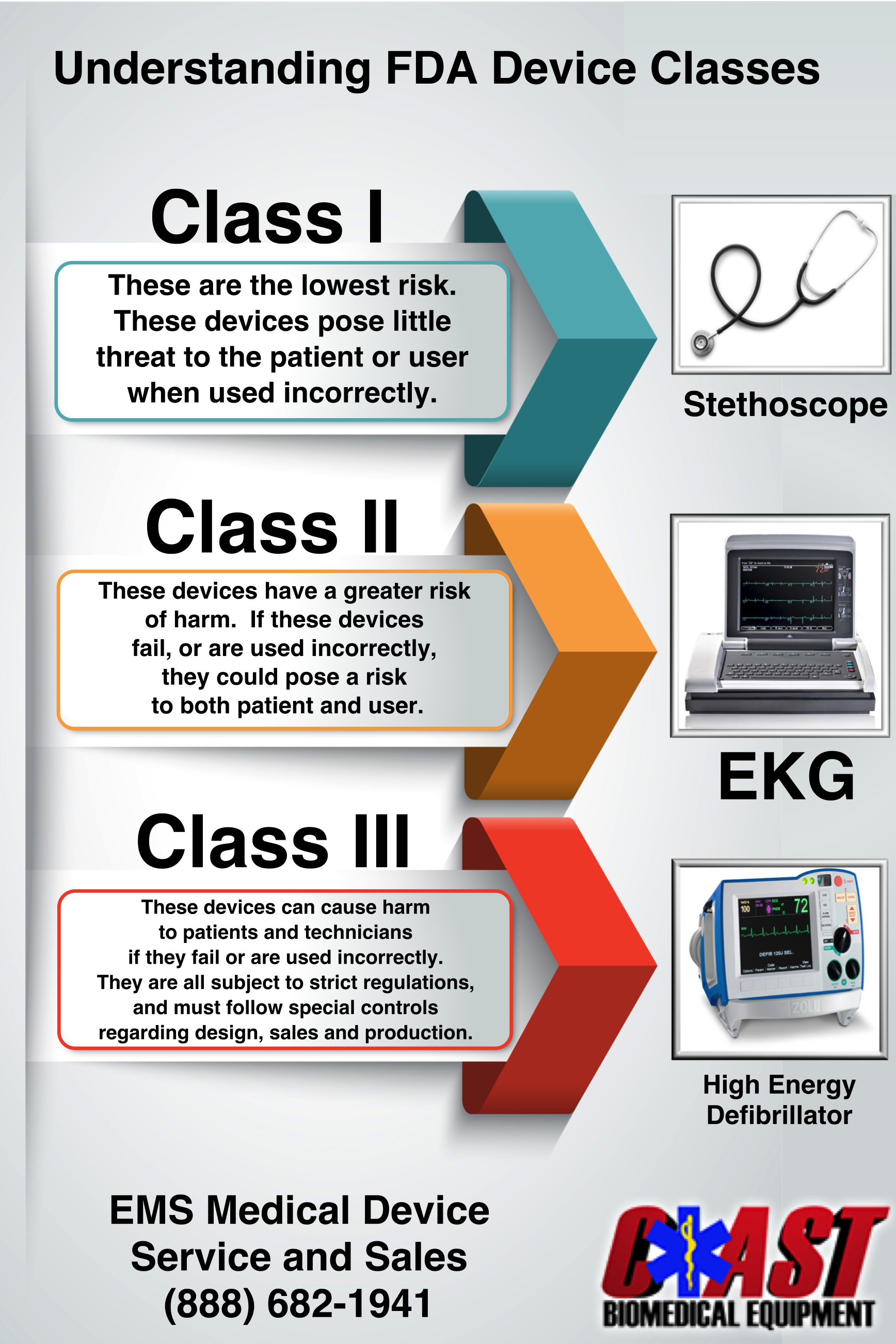
Blog Coast Biomedical Equipment
https://coastbiomed.com/wp-content/uploads/2016/01/CoastInfographic.png

Understanding EBike Classification Electric Bike Journal
https://www.electricbikejournal.com/wp-content/uploads/2023/03/EBJ_Youtube_Thumbnail.jpg

Class 1 Confined Space Polygon Singing Rock
https://www.polygon-singingrock.com/m-images/1/1062/638124826411633333/preview/l-1502x2000-85/IMG_20221026_141932.jpg
When it comes to the major differences between a class one medical device and a class two medical device the most significant differences come down to the potential risk to the user and the complexity of the device The more complex Class II medical devices are considered to have a higher risk level compared to Class I devices They are more complex in design and function and they require a higher level of regulatory control Examples of Class II medical devices
Explore the crucial distinctions between a Class I medical device Class II and Class III while understanding their characteristics and regulatory nuances Class I devices are the least regulated and comprise about 47 of all FDA registered medical devices Approximately 95 of these devices are exempt from the
Class III Device Definition Arena
https://www.arenasolutions.com/wp-content/uploads/ClassThreeDevice-1080x789.png

Go Chair MED FDA Class II Medical Device Mediplus Mobility
https://www.mediplusmobility.com/wp-content/uploads/2020/11/GoChair-Med-Right-Beauty-8-20-scaled.jpg

https://www.fda.gov › medical-devices › overview...
Class I includes devices with the lowest risk and Class III includes those with the greatest risk As indicated above all classes of devices as subject to General Controls

https://www.compliancegate.com › medica…
The Medical Devices Regulation sets requirements according to device class More specifically medical devices are defined as class I IIa IIb or III In turn this determines certain requirements and which conformity
Circuit 1

Class III Device Definition Arena
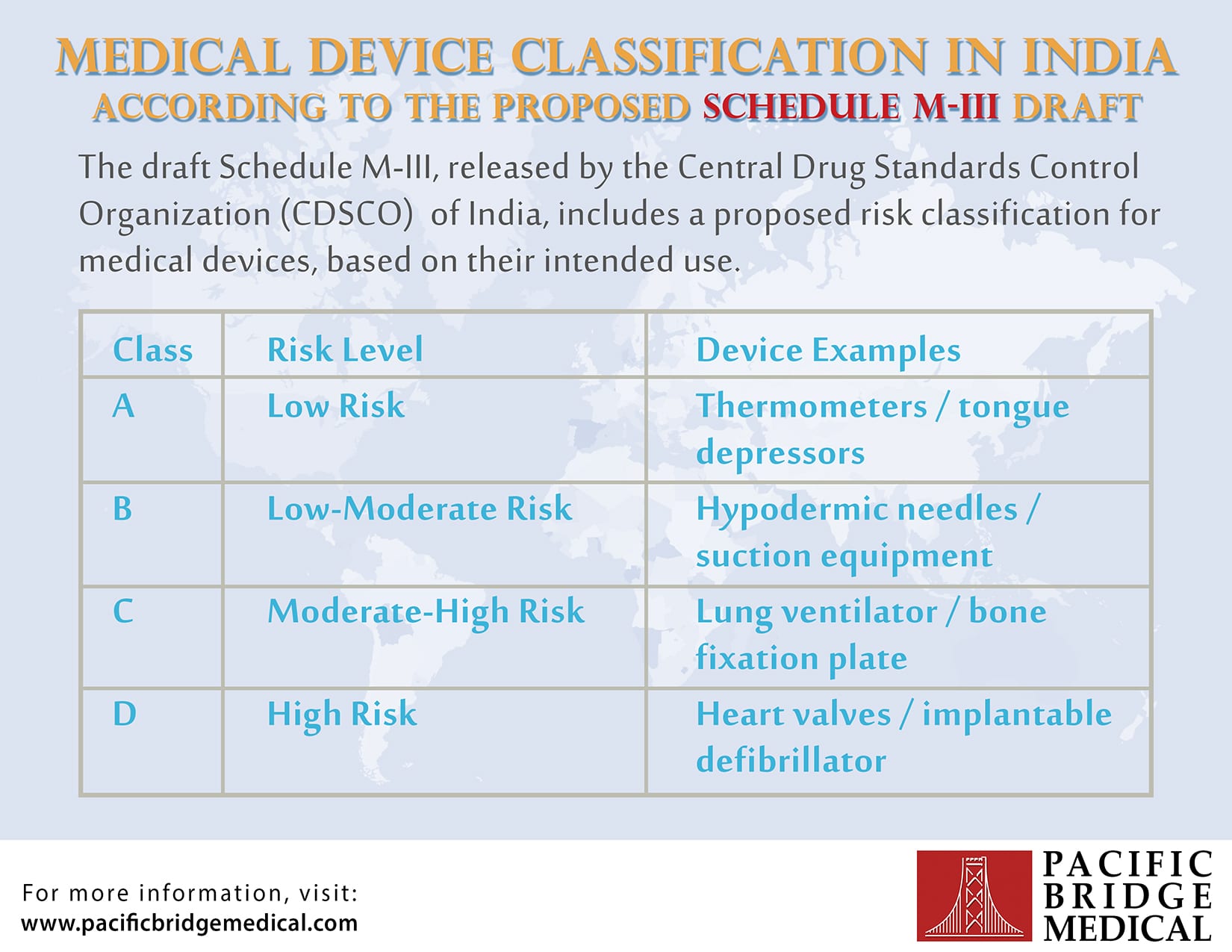
Device Classification In India Infographic
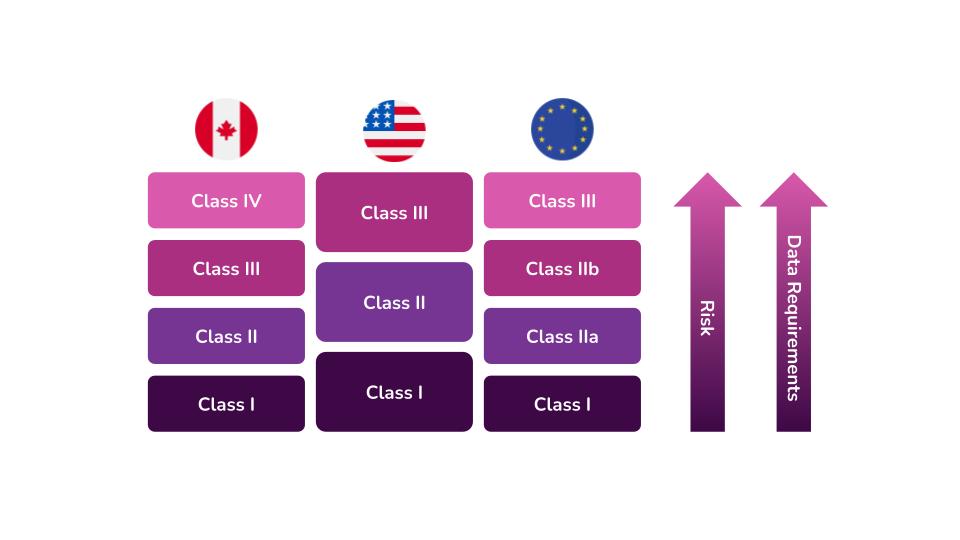
FDA Class II Medical Devices

Ca Biomedical Devices
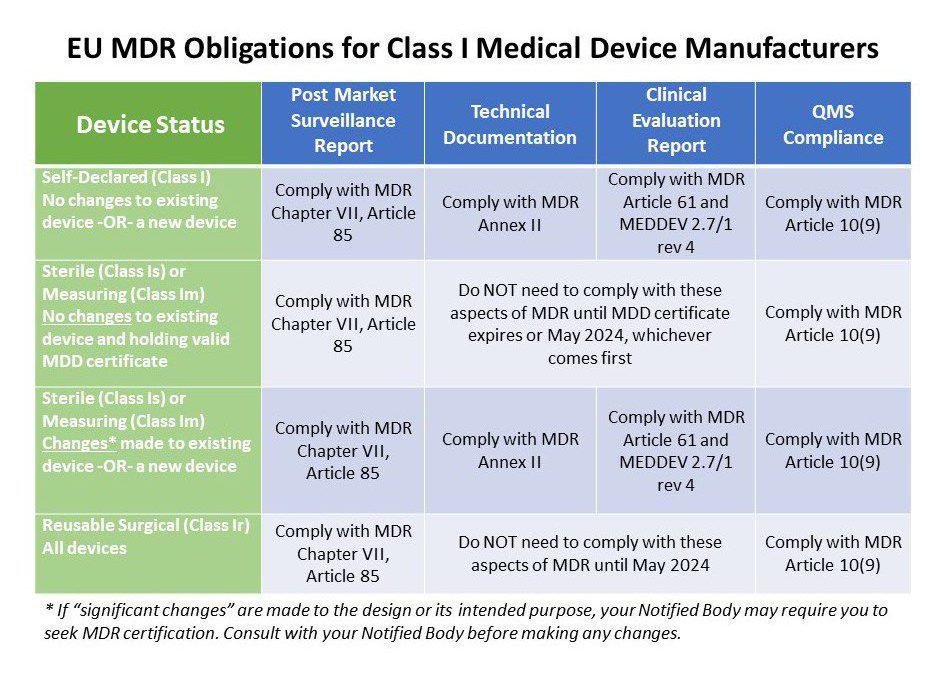
Class 1 Medical Device Requirements Oriel STAT A MATRIX

Class 1 Medical Device Requirements Oriel STAT A MATRIX
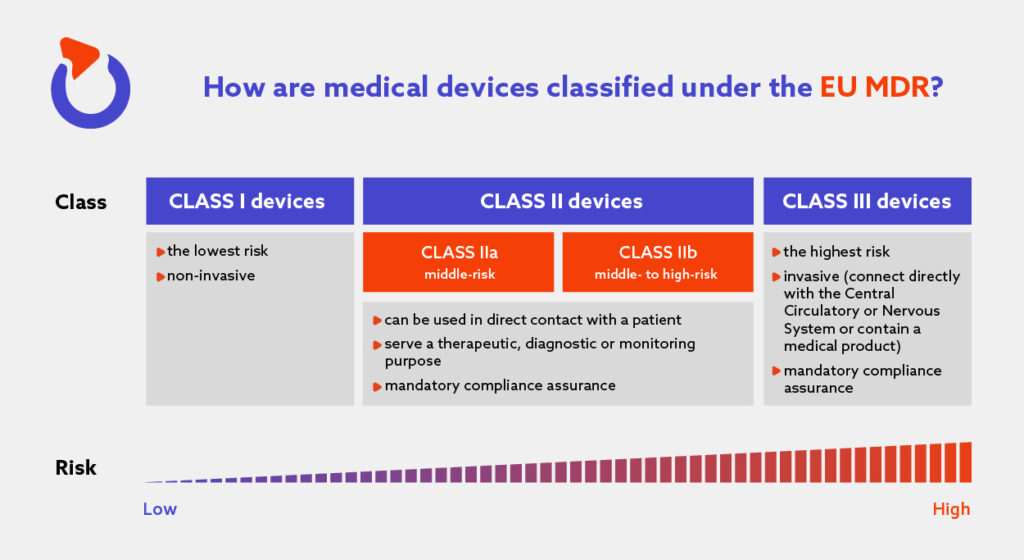
The Complete Guide To EU Medical Device Regulation Spyrosoft

Apple Watch LIHKG
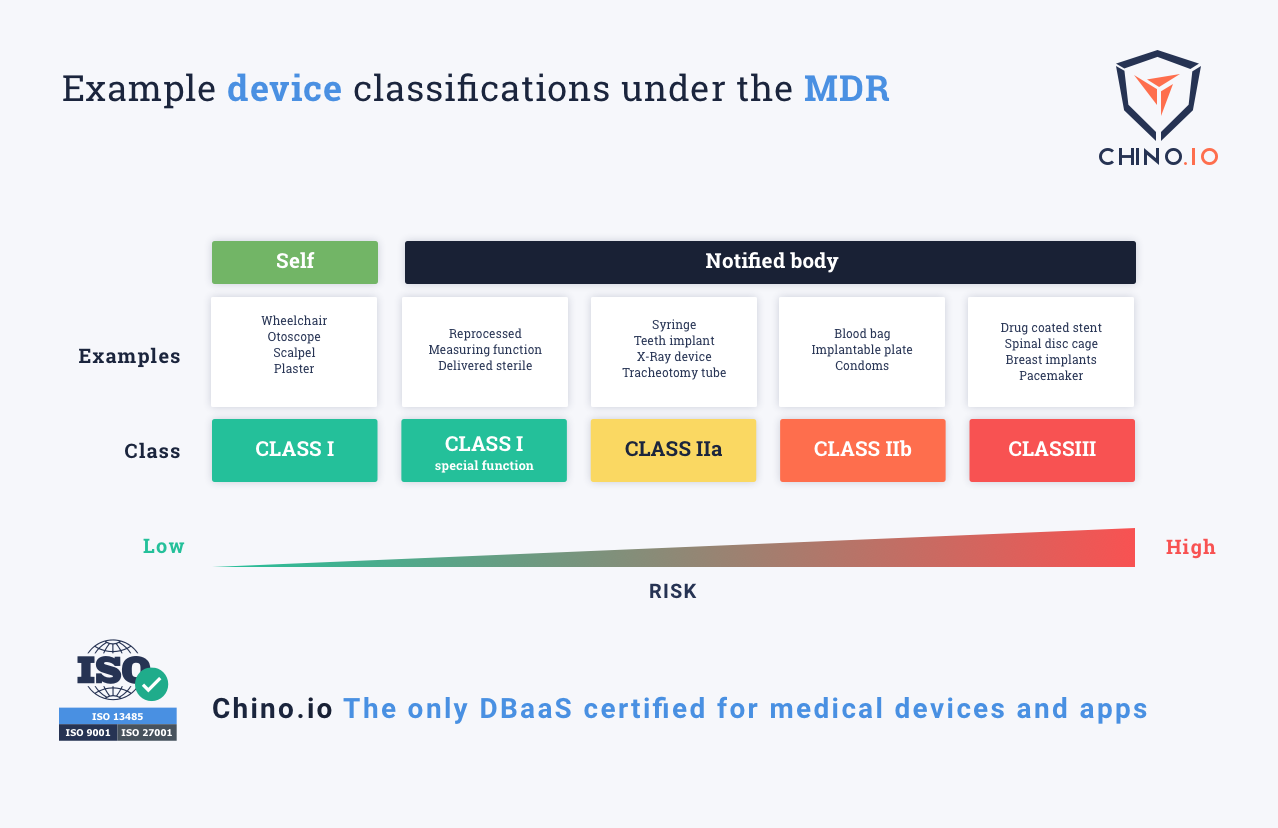
What MDR Class Is My EHealth App
Class 1 Vs Class 2 Medical Device - Class I Class I medical devices pose a minimal danger to patients and are primarily used for non invasive procedures and basic functions Class IIa Class IIa devices