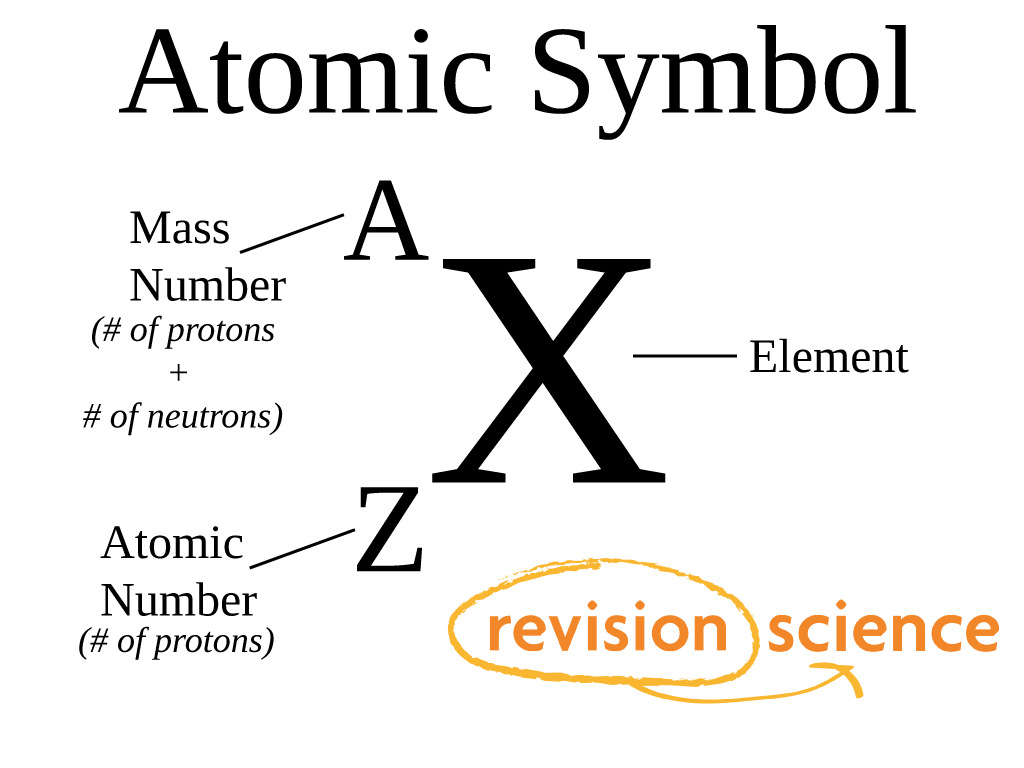
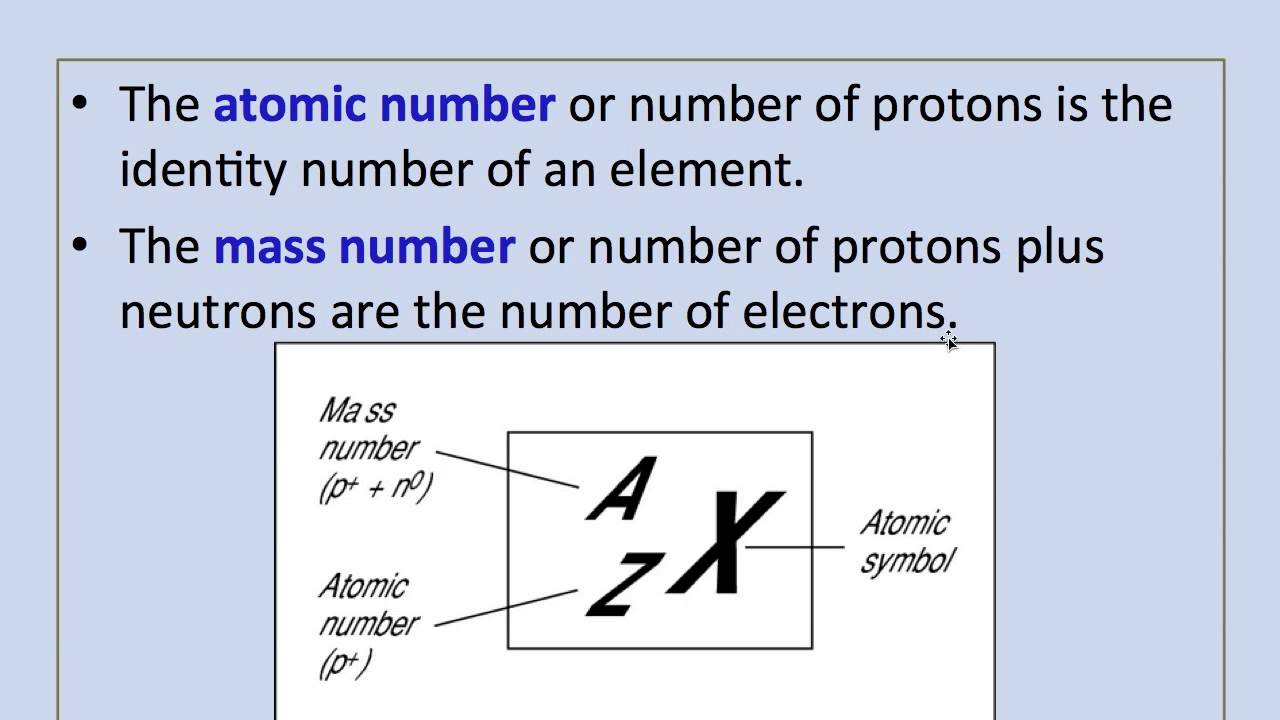
Mass Number Definition Mass number is an integer whole number equal to the sum of the number of protons and neutrons of an atomic nucleus In other words it is the sum of the number of nucleons in an atom Mass number is often denoted using a capital letter A
Mass number in nuclear physics the sum of the numbers of protons and neutrons present in the nucleus of an atom The mass number is commonly cited in distinguishing among the isotopes of an element all of which have the same atomic number The mass number is the total number of protons and neutrons present inside the nucleus of an atom It represents the mass of the atom Mass number Number of protons Number of neutrons
Mass Number Definition
Mass Number Definition
https://1.bp.blogspot.com/-vGmcj1SyVqA/YBuuf5t6UbI/AAAAAAAAAV8/JskWl0v1xP4cYw9YMncvEOHtE73M5cf6gCLcBGAsYHQ/s762/definition%2Bof%2Bmass%2Bnumber.PNG

Mass Number Definition Equation And Examples Chemistry YouTube
https://i.ytimg.com/vi/XlWONPrpdJE/maxresdefault.jpg
:max_bytes(150000):strip_icc()/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
Mass And Atomic Mass Number
https://www.thoughtco.com/thmb/vSpe3Hy58nZeJ4tZQ8_fComvX7I=/1500x0/filters:no_upscale():max_bytes(150000):strip_icc()/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png
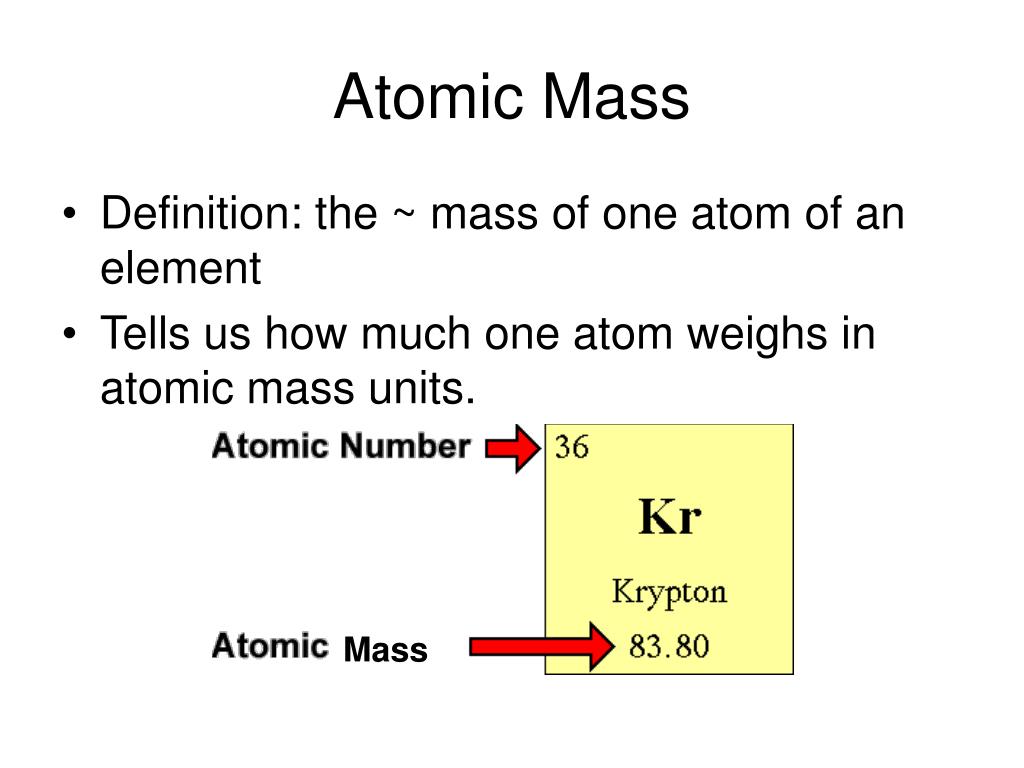
What is the Mass Number The atomic number of an element is determined by the number of protons in it and it is used to differentiate one element from another The mass number of an element is determined by the number of protons and neutrons combined The atomic number of a sodium atom is 11 and its mass number is 23 Calculate the number of protons neutrons and electrons it contains
The mass number is the total number of proteins and neutrons in an atom Elements have isotopes with the same atomic number but the different mass number The average atomic mass of isotopes is called relative atomic mass Mass number is the sum of the number of protons and neutrons in an atom In other words it is the number of nucleons in an atom Mass number is also a whole number with the symbol A in general notation It is given on the upper right or upper left side of
More picture related to Mass Number Definition
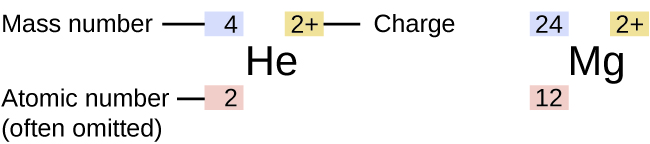
Mass Number Example
https://alevelchemistry.co.uk/wp-content/uploads/2020/06/1.-Mass-Number.png

How To Write Atomic
https://sciencenotes.org/wp-content/uploads/2020/10/atomic-and-mass-number.jpg
/chlorine--chemical-element--186451006-5ad48c0ffa6bcc0036b60abd.jpg)
Mass Number Definition And Examples
https://www.thoughtco.com/thmb/OCFobhrOlRqTFkyLdl3HTo54t4Q=/5370x3580/filters:fill(auto,1)/chlorine--chemical-element--186451006-5ad48c0ffa6bcc0036b60abd.jpg
The mass number represented by the letter A is defined as the total number of protons and neutrons in an atom Consider the table below which shows data from the first six elements of the periodic table Consider the element helium Its atomic number is 2 so it has two protons in its nucleus Its nucleus also contains two neutrons It is defined as the sum of protons and neutrons The mass number is almost equal to the atomic mass of a particular atom Therefore it can be written as Mass no of an atom No of protons No of neutrons Thus it represents the total number of neutrons present in the nucleus of an atom Mass number helps to give an idea of the isotopic mass
[desc-10] [desc-11]
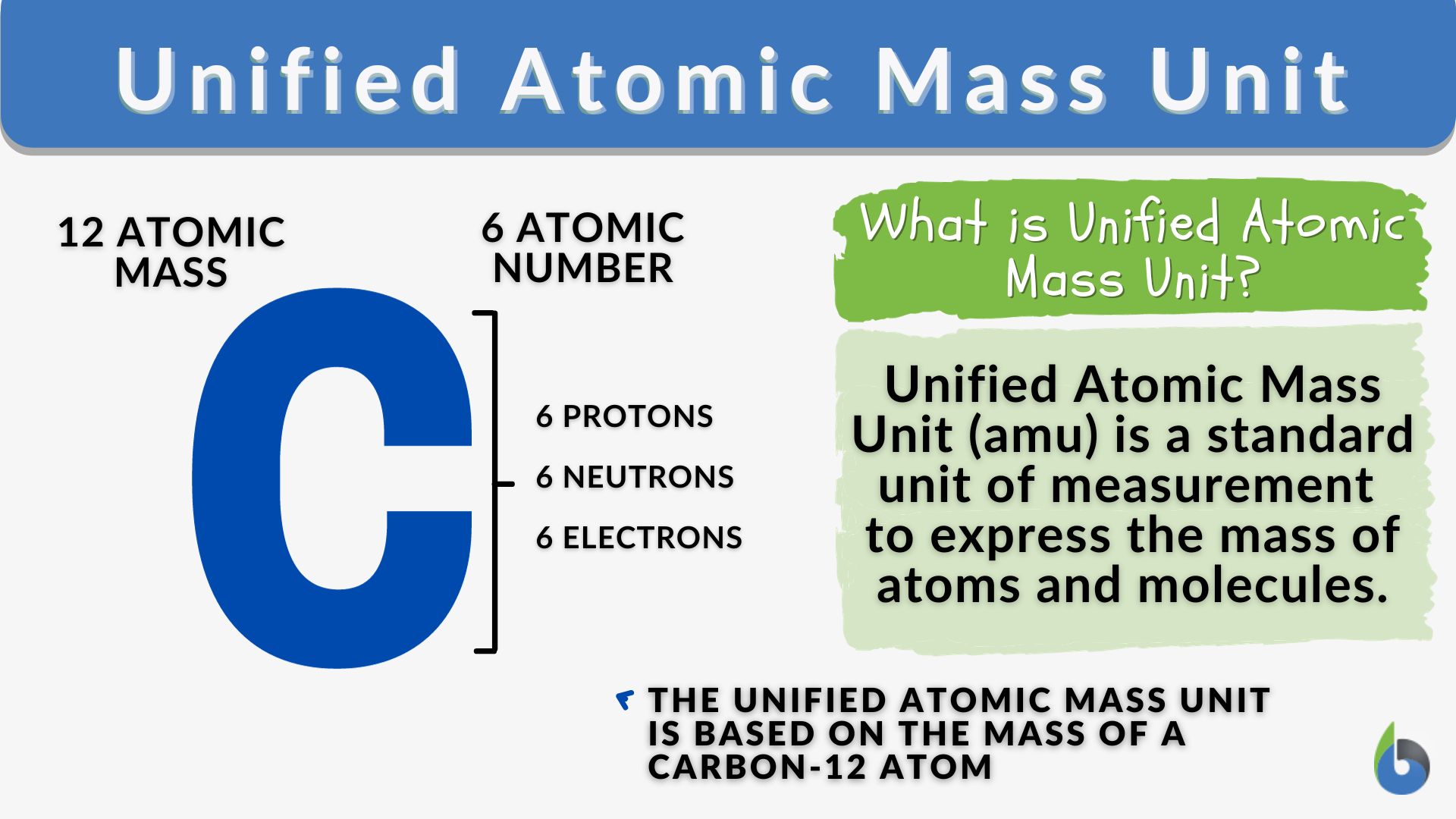
Unified Atomic Mass Unit Definition And Examples Biology Online
https://www.biologyonline.com/wp-content/uploads/2019/10/unified-atomic-mass-unit-definition-and-example.jpg

What Is An Atomic Number Definition And Examples Atomic Number
https://i.pinimg.com/originals/c6/ac/21/c6ac2194f1deb3897e17afd90ad66579.png
https://www.thoughtco.com/definition-of-mass-number-604564
Mass number is an integer whole number equal to the sum of the number of protons and neutrons of an atomic nucleus In other words it is the sum of the number of nucleons in an atom Mass number is often denoted using a capital letter A

https://www.britannica.com/science/mass-number
Mass number in nuclear physics the sum of the numbers of protons and neutrons present in the nucleus of an atom The mass number is commonly cited in distinguishing among the isotopes of an element all of which have the same atomic number
Question fab79 Socratic

Unified Atomic Mass Unit Definition And Examples Biology Online
PPT Organization Of The Periodic Table PowerPoint Presentation Free
Maxresdefault Dynamic Periodic Table Of Elements And Chemistry

Mass Number Easy Science Mass Number Easy Science Number Definition

Relative Atomic Mass OCR A Level Teaching Resources

Relative Atomic Mass OCR A Level Teaching Resources

Mass Number Definition Overview Expii

Mass Number Versus Atomic Number And Atomic Mass

How To Calculate The Mass Number
Mass Number Definition - [desc-12]