Parafuso 1 4 X 110 Notably both heat capacity and specific heat capacity involve the change in temperature of a substance without a change in the state solid liquid and gas of the substance
The thermal properties of water included in this lesson include its thermal conductivity specific heat capacity boiling point melting point heat of fusion and density Learn how heat is measured Understand the heat measurement unit heat capacity of a calorimeter and specific heat of some substances See how
Parafuso 1 4 X 110

Parafuso 1 4 X 110
https://www.eletricagorec.com.br/wp-content/uploads/2021/08/IMG_2273-1-600x600.jpg

PARAFUSO PARA MADEIRA JOMARCA Paes Leme Parafusos E Ferragens
https://paeslemeparafusos.com.br/wp-content/uploads/2021/12/1007-2.jpg
Parafuso Sextavado Rosca Soberba 1 4 3 16 5 16 100 PE AS Shopee
https://down-br.img.susercontent.com/file/br-11134207-7qukw-lh3sv8vpiieu5c
A metal s heat capacity can absorb thermal energy before a one unit change in temperature is observed Metals have low heat capacity values when compared to water What is Molar Specific Heat Heat capacity is the amount of heat energy absorbed or released by a chemical substance per the change in temperature of that substance The change in heat is
Learn how to calculate the specific heat of a substance and see examples that walk through sample problems step by step for you to improve your physics knowledge and skills Newton s Law of Cooling states that the rate of loss of heat or the cooling rate of a body is directly proportional to the temperature difference between the body and the
More picture related to Parafuso 1 4 X 110
Parafuso Polegada Para Mm EDUCA
https://img.lojadomecanico.com.br/IMAGENS/37/466/145325/Parafuso-Sextavado-516-Pol-x-112-Pol-com-200-unid-7898338094715-1.JPG
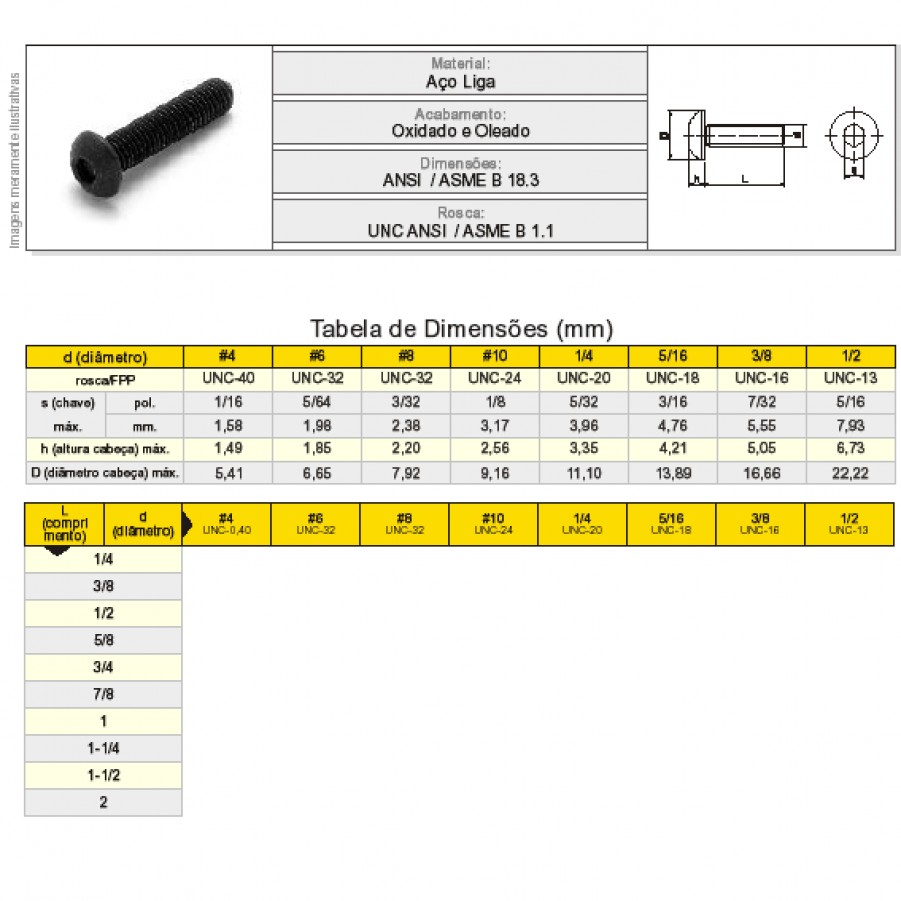
Parafuso Allen Com Sextavado Interno A o Liga Cabe a Abaulada Rosca NC
https://www.reiparparafusos.com.br/image/cache/data/produtos/16-08-2017/7-parafuso-allen-com-sextavado-interno-aco-liga-cabeca-abaulada-nc-901x901.jpg

Parafuso Sextavado Inox 304 UNC 5 16 X 3 4 Ciser
https://static3.tcdn.com.br/img/img_prod/841507/parafuso_sextavado_inox_304_unc_5_16_x_3_4_ciser_9061_1_20200924120935.jpg
The specific heat capacity of water is 4 180 kilojoules per kilogram kelvin kJ kg K Determine the temperature in degrees Celsius C of the water as it exits the boiler A diatomic ideal gas at What is Debye model theory In solid state physics debye theory is used to estimate the phonons contributing to the specific heat capacity in a solid It explains that the specific heat is a
[desc-10] [desc-11]

Parafuso Chipboard Flangeada Fenda Phillips CRV Industrial Parafusos
https://www.crvindustrial.com/admin/files/produto/original__508fed40_parafuso_chipboard_flangeada_fenda_phillips_01.jpg
10 Parafuso Com Porca E Arruela V rios Tamanhos Com Cabe a Fenda Kit
https://down-br.img.susercontent.com/file/7614cc5361deac3b4f064be439a69a75

https://study.com › academy › lesson › how-to-calculate-specific-heat-ca…
Notably both heat capacity and specific heat capacity involve the change in temperature of a substance without a change in the state solid liquid and gas of the substance

https://study.com › academy › lesson › thermal-properties-of-water.html
The thermal properties of water included in this lesson include its thermal conductivity specific heat capacity boiling point melting point heat of fusion and density

Parafuso Sextavado Rosca Inteira ANSME B 18 2 1 DIN 933 CRV

Parafuso Chipboard Flangeada Fenda Phillips CRV Industrial Parafusos

Parafusos Com Porca E Arruela 1 4 5 16 3 8 Parafuso Sextavado Rosca

Chumbador Parabolt Parafuso 1 4 X 2 ncora Kit Com 10 P s MercadoLivre

Parafuso Allen Cabe a Cil ndrica Inox 304 M12 1 75 X 80 CCP Virtual

Parafuso Franc s Com Porca 1 4 X 3 Polegadas Parafusos Projette

Parafuso Franc s Com Porca 1 4 X 3 Polegadas Parafusos Projette

Parafuso Auto Brocante Cabe a Sextavada C Arruela De Veda o P Telha

Parafuso Sextavado Ferro Zincado Rosca Parcial

Parafuso Allen Com Cabe a Chata A o Liga 7 16 14 X 1 1 2 Unc CCP
Parafuso 1 4 X 110 - [desc-13]


