What Is Boiling Point The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid 1 2 and the liquid changes into a vapor The boiling point of a liquid varies depending upon the surrounding environmental pressure
The boiling point is the temperature for a particular liquid to boil at For example the boiling point for water at a pressure of 1 atm is 100 degrees Celsius A liquid s boiling point depends upon the liquid s temperature atmospheric pressure and vapor pressure The formal definition in science is that boiling point is the temperature where the vapor pressure of a liquid equals the vapor pressure of its environment At this temperature the liquid changes into the vapor gas phase In both boiling and evaporation a
What Is Boiling Point
What Is Boiling Point
https://engineeringstuff.co.in/wp-content/uploads/2020/04/InkedCapture_LI.jpg

How To Remember Melting Point And Boiling Points Of Full Periodic Table
https://i.ytimg.com/vi/vfN0dmmFSbg/maxresdefault.jpg
Boiling Point Definition Image GameSmartz
https://gamesmartz.com/upload/subjects/science/800/boiling-point.png
But the boiling point of water changes with elevation The boiling point is a higher temperature below sea level and a lower temperature above sea level The boiling point of water is the temperature where the liquid s vapor pressure equals atmospheric pressure The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid Therefore the boiling point of a liquid depends on atmospheric pressure The boiling point becomes lower as the external pressure is reduced
The boiling point is the temperature at which boiling occurs for a specific liquid For example for water the boiling point is 100 C at a pressure of 1 atm The boiling point of a liquid depends on temperature atmospheric pressure and the vapor pressure of the liquid Normally when we boil a liquid we do so at atmospheric pressure If this pressure is the standard pressure of 1 atm 101 3 kPa then the temperature at which the liquid boils is referred to as its normal boiling point This is the boiling point which is usually quoted in chemical literature Not everyone lives at sea level though
More picture related to What Is Boiling Point

What Is Boiling Point Concept Of Boiling Point Boiling Point
https://i.ytimg.com/vi/ce9enQmFujc/maxresdefault.jpg

Boiling Point Definition Of Boiling Point
https://www.healthbenefitstimes.com/glossary/wp-content/uploads/2020/07/Boiling-Point.jpg

Large Molecules High Boiling Point Not Very Volatile Sale
https://sciencenotes.org/wp-content/uploads/2021/11/Boiling-Point-Elevation.png
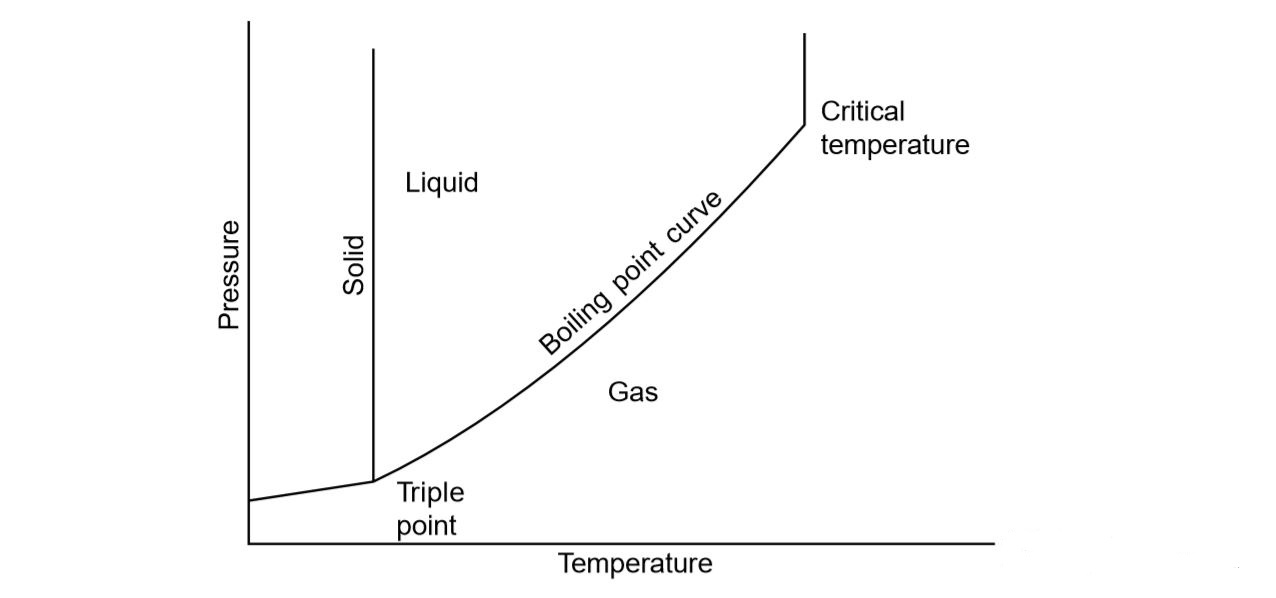
Boiling point is a temperature at which the vapor pressure is equal to the surrounding pressure Boiling Point is defined as the temperature at which matter changes from liquid to gaseous state Some the characteristics of boiling point of At this point the liquid begins to boil The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure The figure below illustrates the boiling of liquid Figure 13 9 2 13 9 2 Comparison between evaporation and boiling
[desc-10] [desc-11]
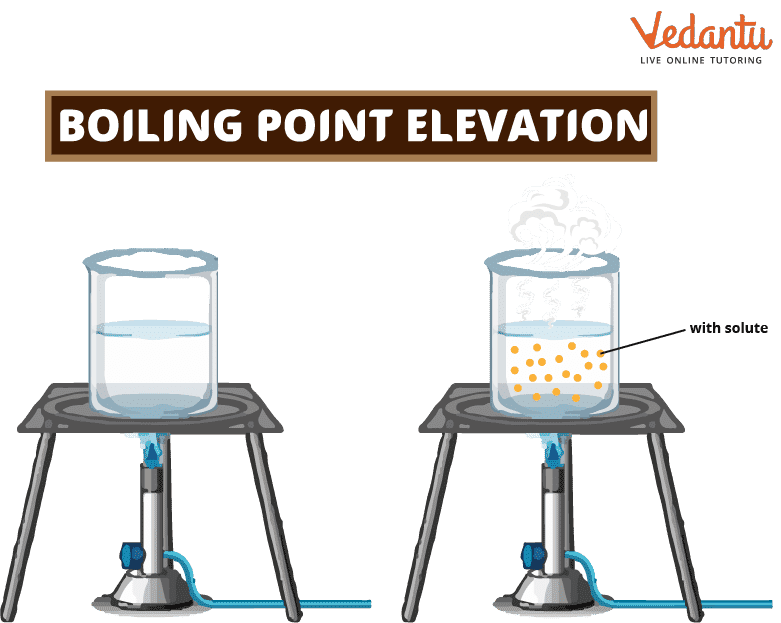
Large Molecules High Boiling Point Not Very Volatile Sale
https://www.vedantu.com/seo/content-images/2e8758b1-dbab-4c94-b565-4c2c924f3bbc.png
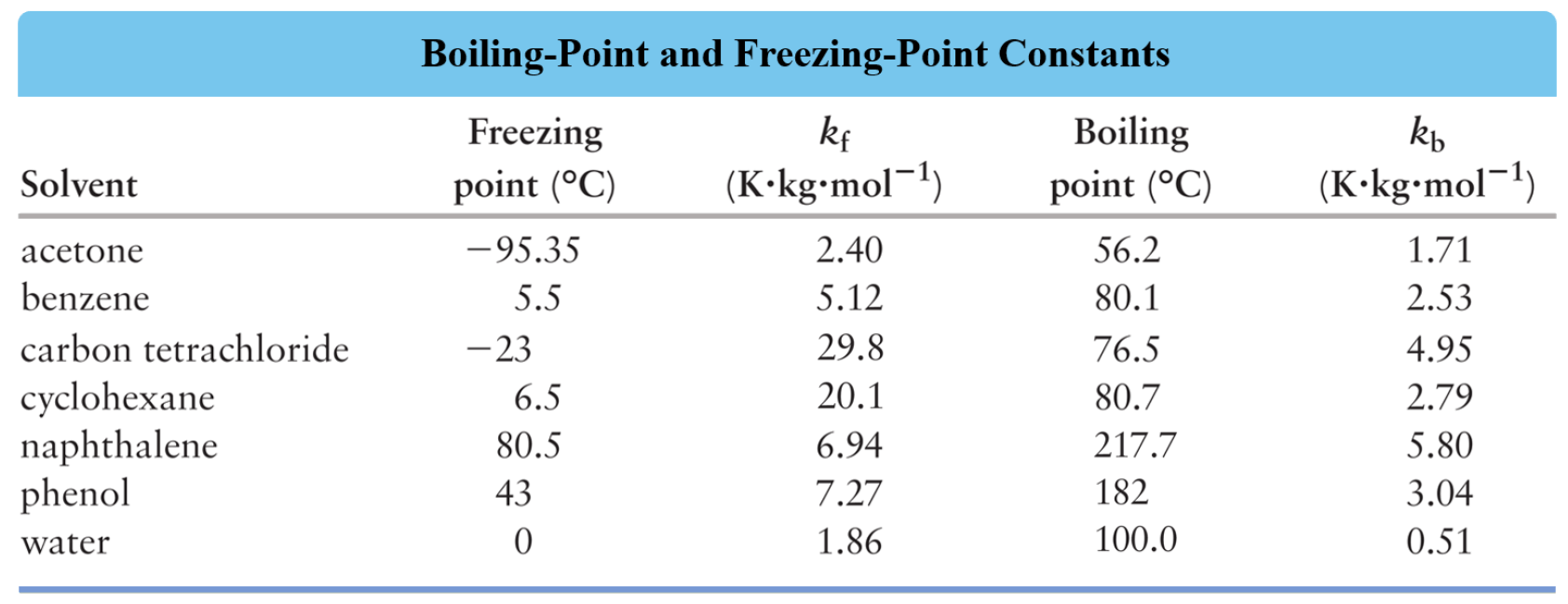
Boiling Point Elevation Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/09/Boiling-point-and-Freezing-point-constants.png
https://en.wikipedia.org › wiki › Boiling_point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid 1 2 and the liquid changes into a vapor The boiling point of a liquid varies depending upon the surrounding environmental pressure

https://byjus.com › chemistry › melting-and-boiling-point
The boiling point is the temperature for a particular liquid to boil at For example the boiling point for water at a pressure of 1 atm is 100 degrees Celsius A liquid s boiling point depends upon the liquid s temperature atmospheric pressure and vapor pressure
/boiling-water-on-gas-stove-143735234-5790aeb35f9b584d2005e949.jpg)
Boiling Point

Large Molecules High Boiling Point Not Very Volatile Sale
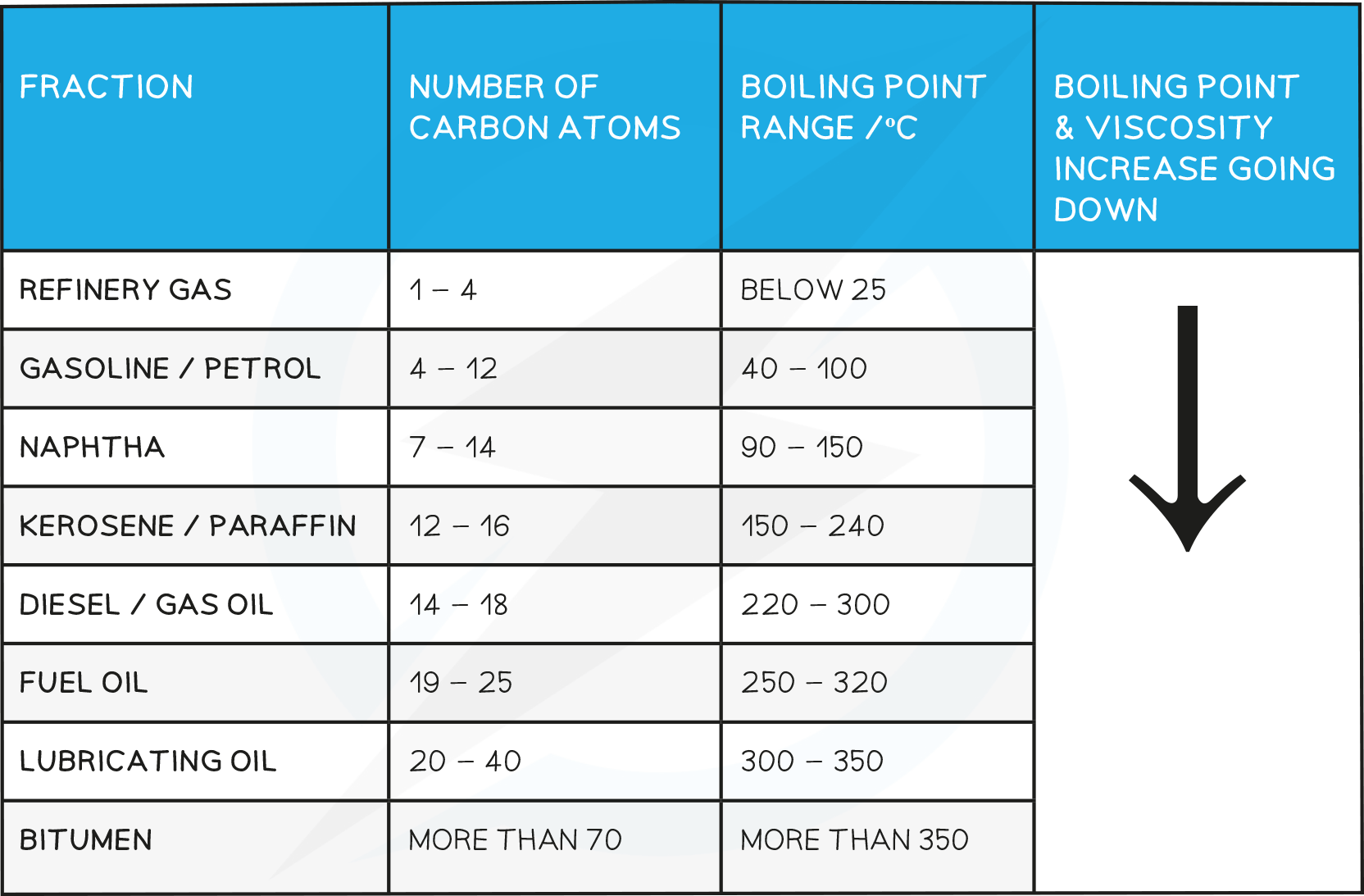
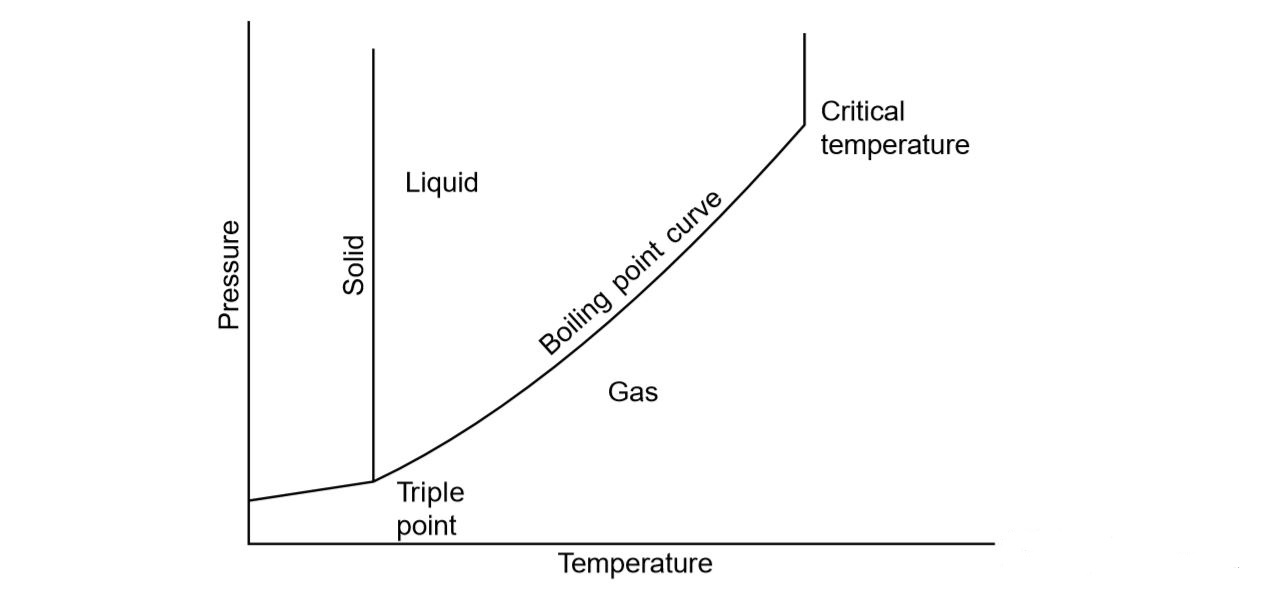
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 4 2 1 Crude Oil

Thermal Secrets To Boiling Point Calibration ThermoWorks

Boiling Point Chemistry
:max_bytes(150000):strip_icc()/boiling-points-of-water-1328760-FINAL-c9c25739167d4722926f2caf69fbae7a.gif)
The Boiling Point Of Water At Various Altitudes
:max_bytes(150000):strip_icc()/boiling-points-of-water-1328760-FINAL-c9c25739167d4722926f2caf69fbae7a.gif)
The Boiling Point Of Water At Various Altitudes
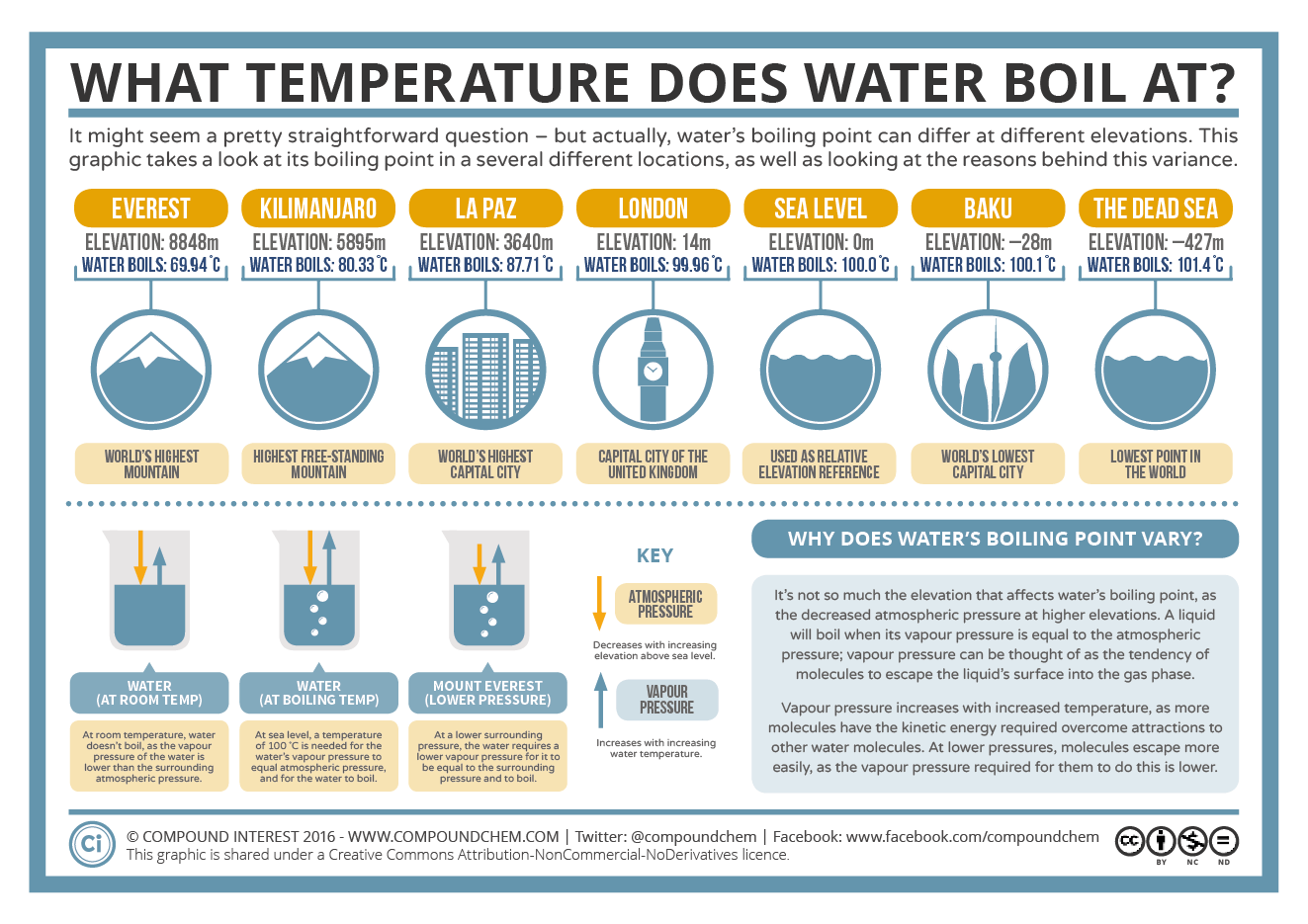
Aprender A Que Temperatura Ferve A gua

Boiling Point
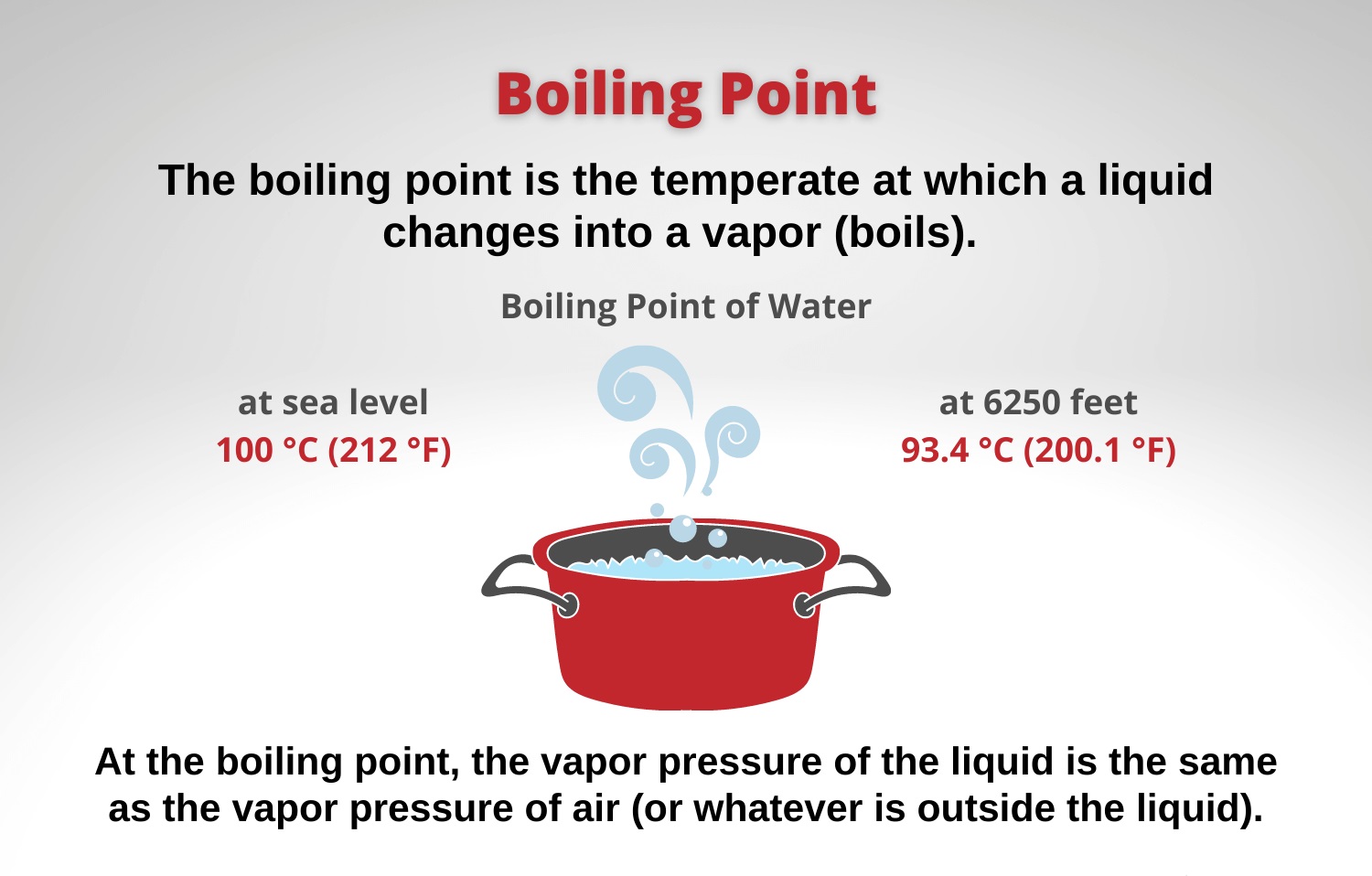
Boiling Point Of Water Fahrenheit
What Is Boiling Point - The boiling point is the temperature at which boiling occurs for a specific liquid For example for water the boiling point is 100 C at a pressure of 1 atm The boiling point of a liquid depends on temperature atmospheric pressure and the vapor pressure of the liquid