What Is The Oxidation Number Of Chlorine In Kclo3 Oxidation occurs when an atom molecule or ion loses one or more electrons in a chemical reaction When oxidation occurs the oxidation state of the chemical species increases
This page discusses the various definitions of oxidation and reduction redox in terms of the transfer of oxygen hydrogen and electrons It also explains the terms oxidizing agent and Oxidation is the process when an atom loses an electron in a reaction with oxygen and water A browned apple or a bicycle is a common phenomenon of oxidation
What Is The Oxidation Number Of Chlorine In Kclo3

What Is The Oxidation Number Of Chlorine In Kclo3
https://i.ytimg.com/vi/g1nW82AU9ac/maxresdefault.jpg

How To Find Oxidation Numbers For Chlorine Cl And Cl2 YouTube
https://i.ytimg.com/vi/MU8xr1vKg8M/maxresdefault.jpg

How To Find The Oxidation Number For Cl In KClO4 Potassium Perchlorate
https://i.ytimg.com/vi/QmITQZMOtq0/maxresdefault.jpg
Oxidation is caused when an atom a molecule or even an ion comes in contact with oxygen When this happens it transfers electrons and changes to get a more stable Define oxidation and how it relates to reduction Learn about redox reaction OIL RIG and the oxidation process Review examples of oxidation Watch the video
OXIDATION definition 1 the process of a substance or chemical element oxidizing 2 the process by which iron and Learn more In an oxidation reaction a substance gains oxygen atoms Learn more in this KS3 Chemistry guide from Bitesize
More picture related to What Is The Oxidation Number Of Chlorine In Kclo3

How To Find The Oxidation Number For Cl In The ClO Ion Hypochlorite
https://i.ytimg.com/vi/GgxUMxoOO_g/maxresdefault.jpg

How To Calculate The Oxidation Number Of Cl In HClO4 perchloric Acid
https://i.ytimg.com/vi/VHTOz5po4dM/maxresdefault.jpg

isequaltoklasses What Is The Oxidation Number Of Chlorine In
https://i.ytimg.com/vi/2gnMqLiwCYs/maxresdefault.jpg
Oxidation is a chemical reaction that involves the loss of electrons by an atom ion or molecule It is a fundamental concept in the study of redox reduction oxidation reactions which are The oxidation state also referred to as the amount of oxidation defines a chemical compound s degree of oxidation loss of electrons of an atom Antoine Lavoisier first used the term
[desc-10] [desc-11]

Oxidation State Examples Online Chemistry Tutor
https://www.onlinechemistrytutor.net/wp-content/uploads/2016/12/Chlorine.001-768x576.jpg

Calculate Oxidation No Of N In NH4 2SO4 Brainly in
https://hi-static.z-dn.net/files/d33/2873a705bb3d13d7a01759b140c9a245.jpg

https://www.thoughtco.com
Oxidation occurs when an atom molecule or ion loses one or more electrons in a chemical reaction When oxidation occurs the oxidation state of the chemical species increases

https://chem.libretexts.org › Bookshelves › Analytical...
This page discusses the various definitions of oxidation and reduction redox in terms of the transfer of oxygen hydrogen and electrons It also explains the terms oxidizing agent and
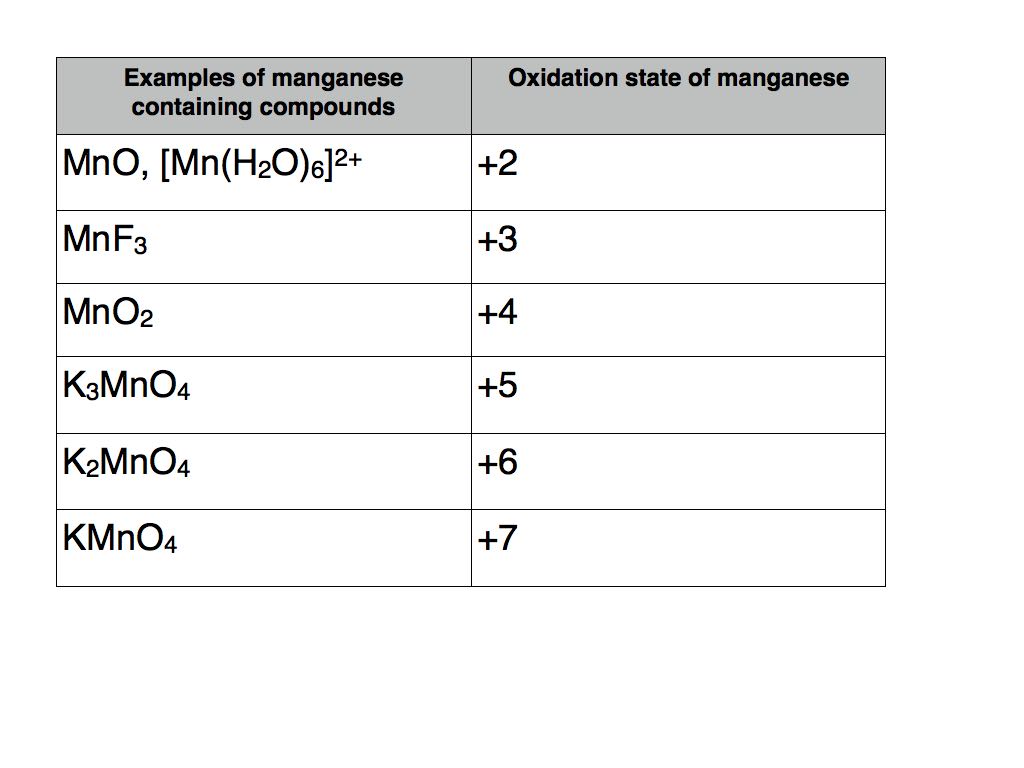
Oxidation State Examples Online Chemistry Tutor

Oxidation State Examples Online Chemistry Tutor

Question Video Determining The Oxidation Number Of Atoms In A Molecule

Define Oxidation Number Find The Oxidation Number Of Carbon In CO2

Oxidation Number State Definition Rules How To Find And Examples

Oxidation Number State Definition Rules How To Find And Examples

Oxidation Number State Definition Rules How To Find And Examples
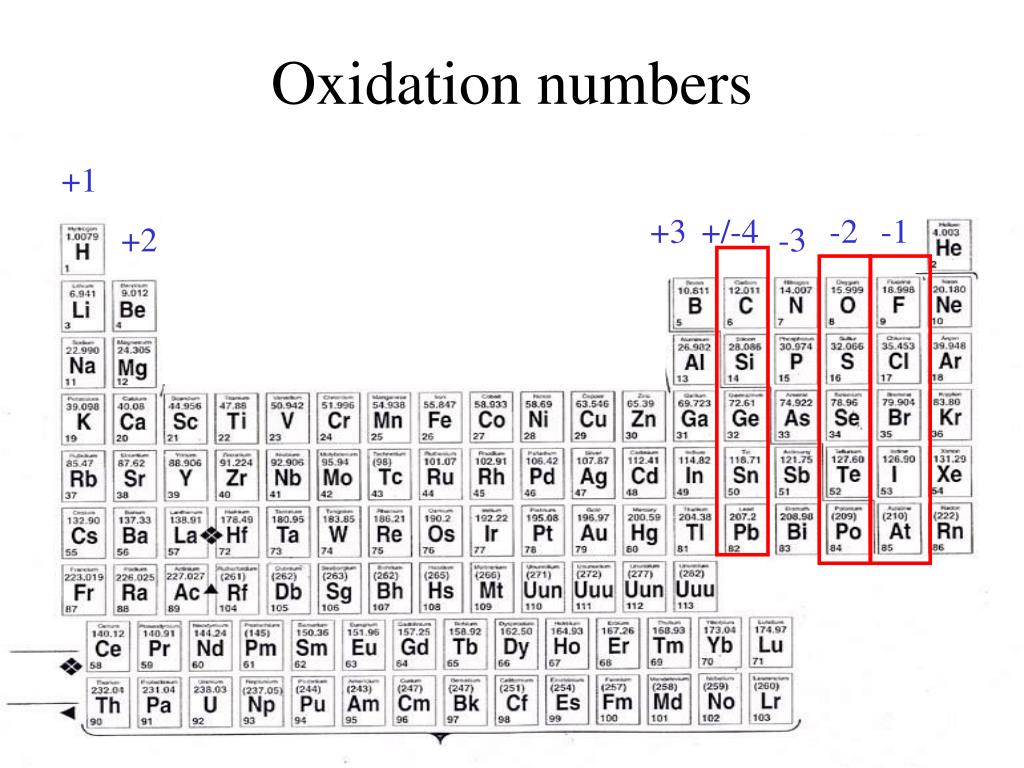
Oxidation Chart Periodic Table Loperstest

Question Video Deducing The Oxidation State Of Oxygen In Hydrogen

FREE Use The Half reactions Of The Reaction Au OH 3 HI Au I2
What Is The Oxidation Number Of Chlorine In Kclo3 - Oxidation is caused when an atom a molecule or even an ion comes in contact with oxygen When this happens it transfers electrons and changes to get a more stable