Which Hybridisation Is Planar The process of mixing of two atomic orbitals of the same atom of similar energy to get a hybrid orbital with equivalent energy and maximum stability and fixed orientation is known as Hybridization
There is a lots of concept is in this question Because if u see the complex u might think about that it will show sp3 hybridisation or dsp2 whatever u r thinking is wrong The metal atom in the complex shows sp2d hybridisation you might not ever heard about the sp2d hybridisation but it is really true Let us see how it is 1 the electronic configuration of copper is Hybridisation number of sigma bonds lone pair CH3Cl 3 sigma bonds between C H and 1 between C and Cl There is no lone pair as carbon has 4 valence electrons and all of them have formed a bond 3 with hydrogen and 1 with Cl
Which Hybridisation Is Planar
Which Hybridisation Is Planar
https://i.ytimg.com/vi/S_729dVqrfs/maxresdefault.jpg

Square Planar Molecular Geometry Shape And Bond Angles YouTube
https://i.ytimg.com/vi/HOirJM3Tjgs/maxresdefault.jpg
KrF2 Lewis Structure How To Draw The Lewis Structure For 44 OFF
https://www.coursehero.com/qa/attachment/3400223/
Read the text carefully and answer the questions In order to explain the characteristic geometrical shapes of polyatomic molecules Pauling introduced the concept of hybridisation The orbitals undergoing hybridisation should have nearly the same energy There are various type of hybridisations involving s p and d type of orbitals In English Definition Hybridization means the intermixing of the atomic orbitals of the same or nearly same energy to produce the new orbitals of exactly the same energy and in same number as b that of the parent orbitals Concept of the Hybridization is only for Sigma bond and lone pair and not for pie bond Types Hybridization is of many types Some of the
Hybridisation Explanation According to VBT half filled atomic orbitals overlap with each other to give completely filled orbitals with pair of electrons this process is known as overlapping of orbitals Thus VBT shows how the pairing of unpaired electron takes place simply depicts the formation of covalent bonds Now for hybridisation of Ti H2O 6 3 oxidation state In Co N H 3 6 3 the oxidation state of cobalt is 3 Ammonia is a strong field ligand so it pair up 4 unpaired electron and free up 2 3 d orbitals These 3 d orbitals are involved in hybridisation with one 4S and three 4P orbitals forming an inner orbital complex so hybridisation of Co N H 3 6 3 is d2sp3 Since it has no unpaired electrons Co N H 3 6 3 is diamagnetic
More picture related to Which Hybridisation Is Planar
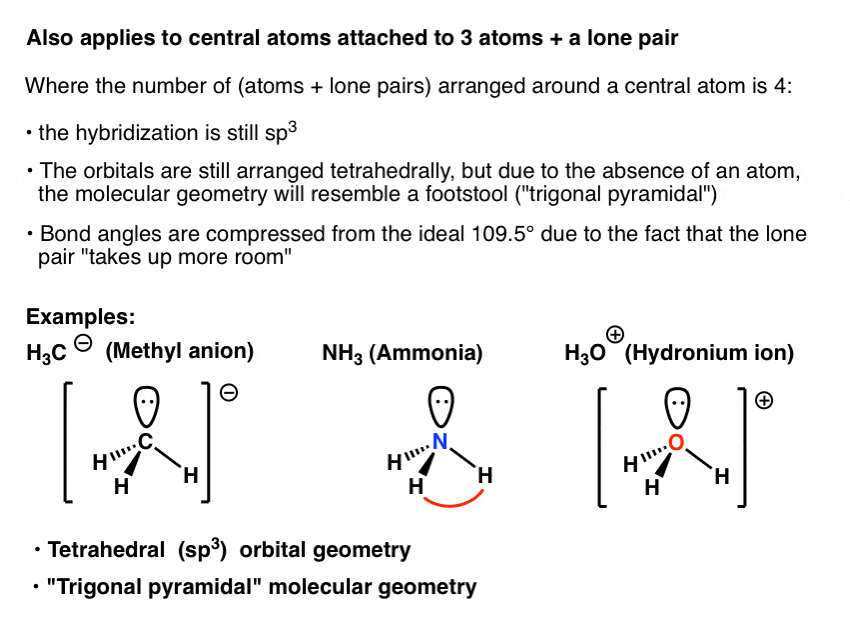
Why Is The Hybridisation In CH3 And CF3 Different Quora 43 OFF
https://cdn.masterorganicchemistry.com/wp-content/uploads/2019/11/7-hybridization-applies-to-central-atoms-attached-to-3-atoms-plus-a-lone-pair-eg-nh3-h3o-and-ch3-trigonal-pyramidal.gif

Hybridization Chart
https://chemistrytalk.org/wp-content/uploads/2023/03/hybridChart-1.png

Hybridization Chart
https://d13loartjoc1yn.cloudfront.net/article/1687420386_Steps to Determine the Type of Hybridization.jpg
Mention the varios methods of inter crop hybridisation No ad blockers please Turn off your ad blocker or try Brainly Plus for no ads Answer Explanation The chemical name of the compound with structure CrO2Cl2 is chromyl chloride It is a coordinate compound with tetrahedral geometry It is an inorganic compound The chromium atom in CrO2Cl2 undergoes d3s hybridisation The oxidation state of chromium in chromyl chloride is 6 The electronic configuration of chromium is given as
[desc-10] [desc-11]
.jpg)
Hybridization Chart
https://d13loartjoc1yn.cloudfront.net/article/1687420524_Shapes of Hybridization (1).jpg

Xef2 Hybridization
https://d1hj4to4g9ba46.cloudfront.net/questions/1959158_1728935_ans_26fa06977adf4697875b69a72b60fe82.jpg
https://www.toppr.com › ask › question › what-is-hybridisation
The process of mixing of two atomic orbitals of the same atom of similar energy to get a hybrid orbital with equivalent energy and maximum stability and fixed orientation is known as Hybridization

https://brainly.in › question
There is a lots of concept is in this question Because if u see the complex u might think about that it will show sp3 hybridisation or dsp2 whatever u r thinking is wrong The metal atom in the complex shows sp2d hybridisation you might not ever heard about the sp2d hybridisation but it is really true Let us see how it is 1 the electronic configuration of copper is
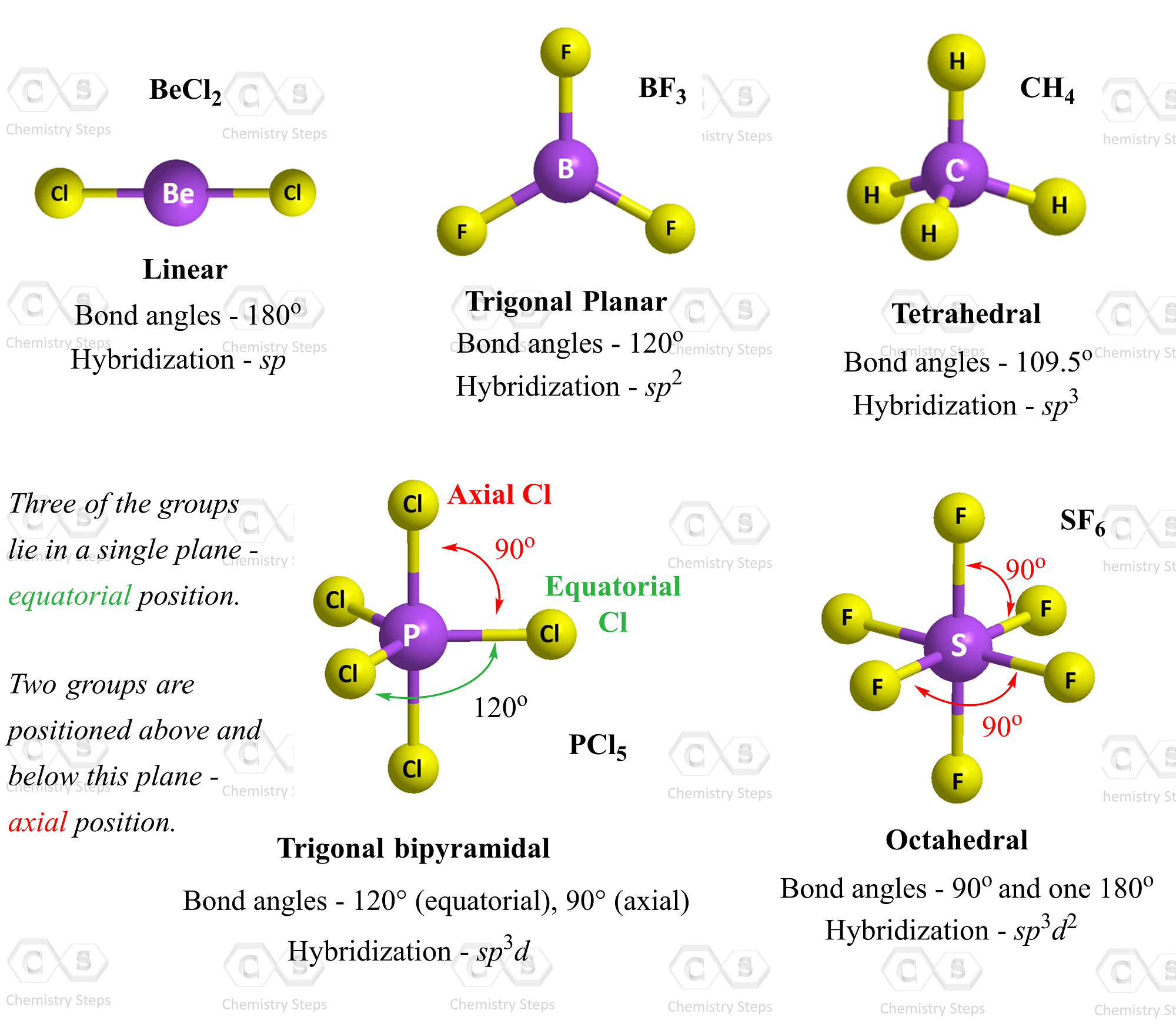
Vsepr Summary Chart
.jpg)
Hybridization Chart
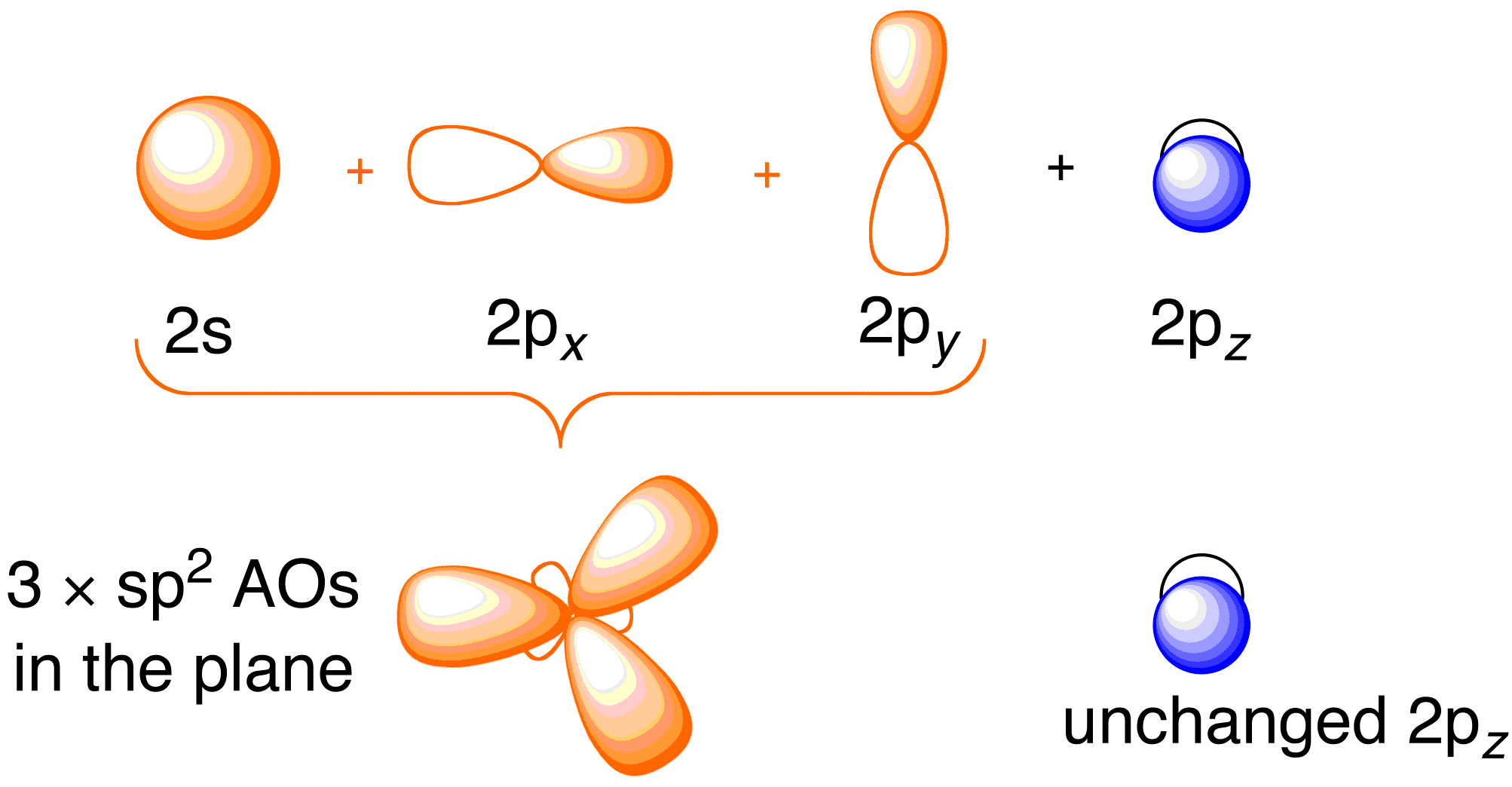
Hybrid Atomic Orbitals Pianovirt
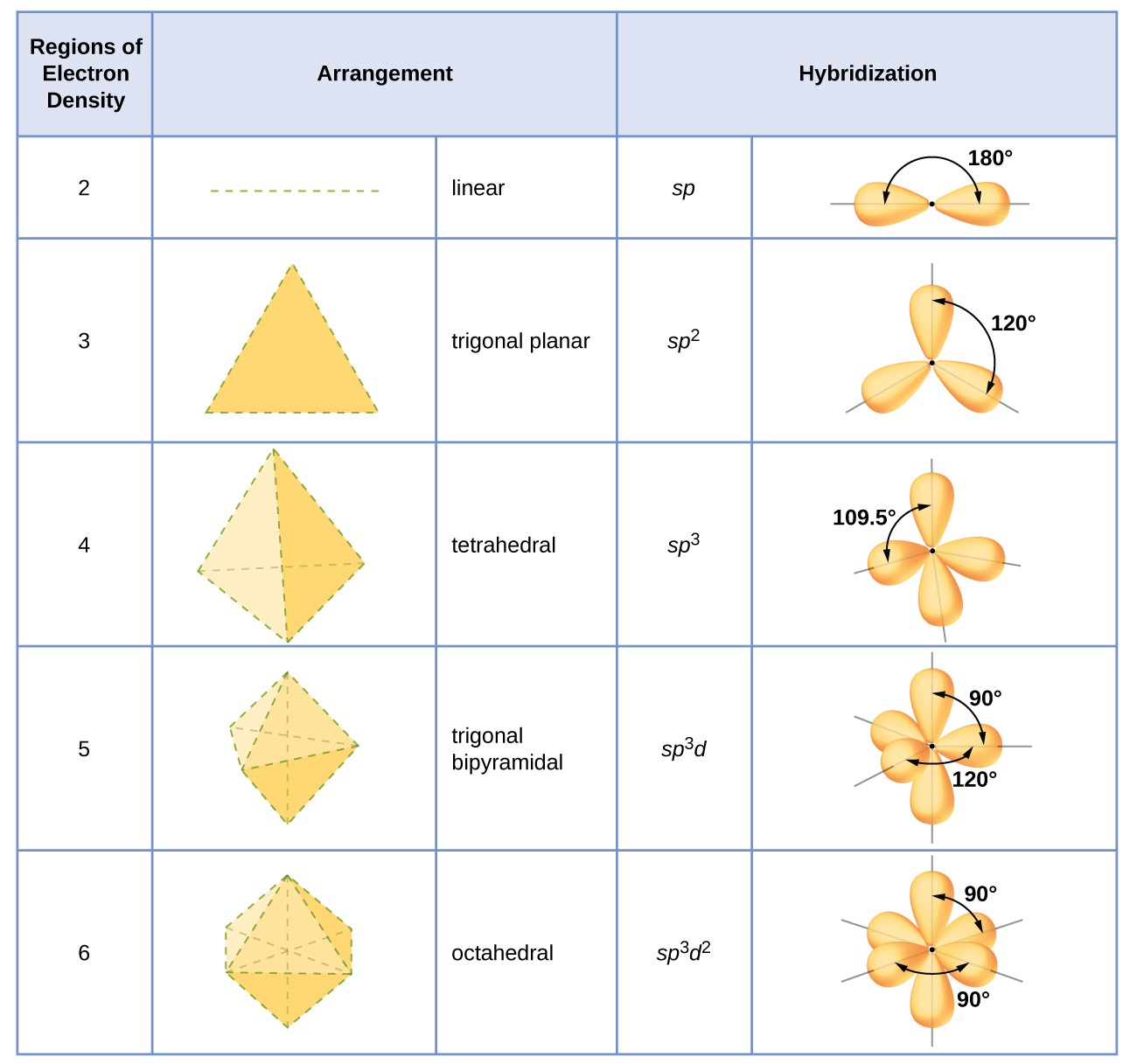
Hybridization Orbitals Chart

Hybridization And Hybrid Orbitals ChemTalk
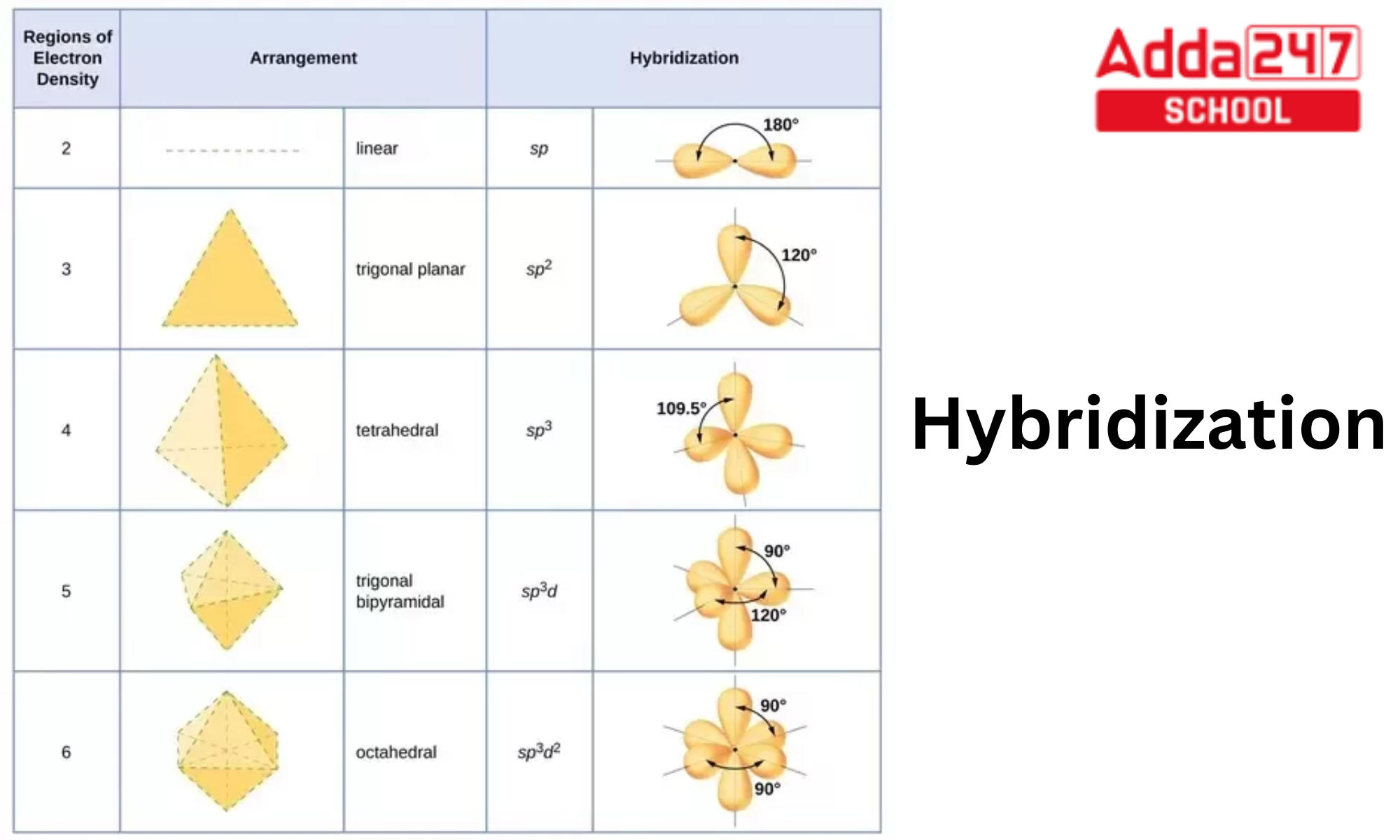
Solidworks 2024 Sp3 Hybridization Deana Adrianne

Solidworks 2024 Sp3 Hybridization Deana Adrianne

C2h2 Hybridization

Trigonal Pyramidal Lewis Structure

Hybridization Of Orbitals Chemistry Topics Chemistry Lessons
Which Hybridisation Is Planar - [desc-14]