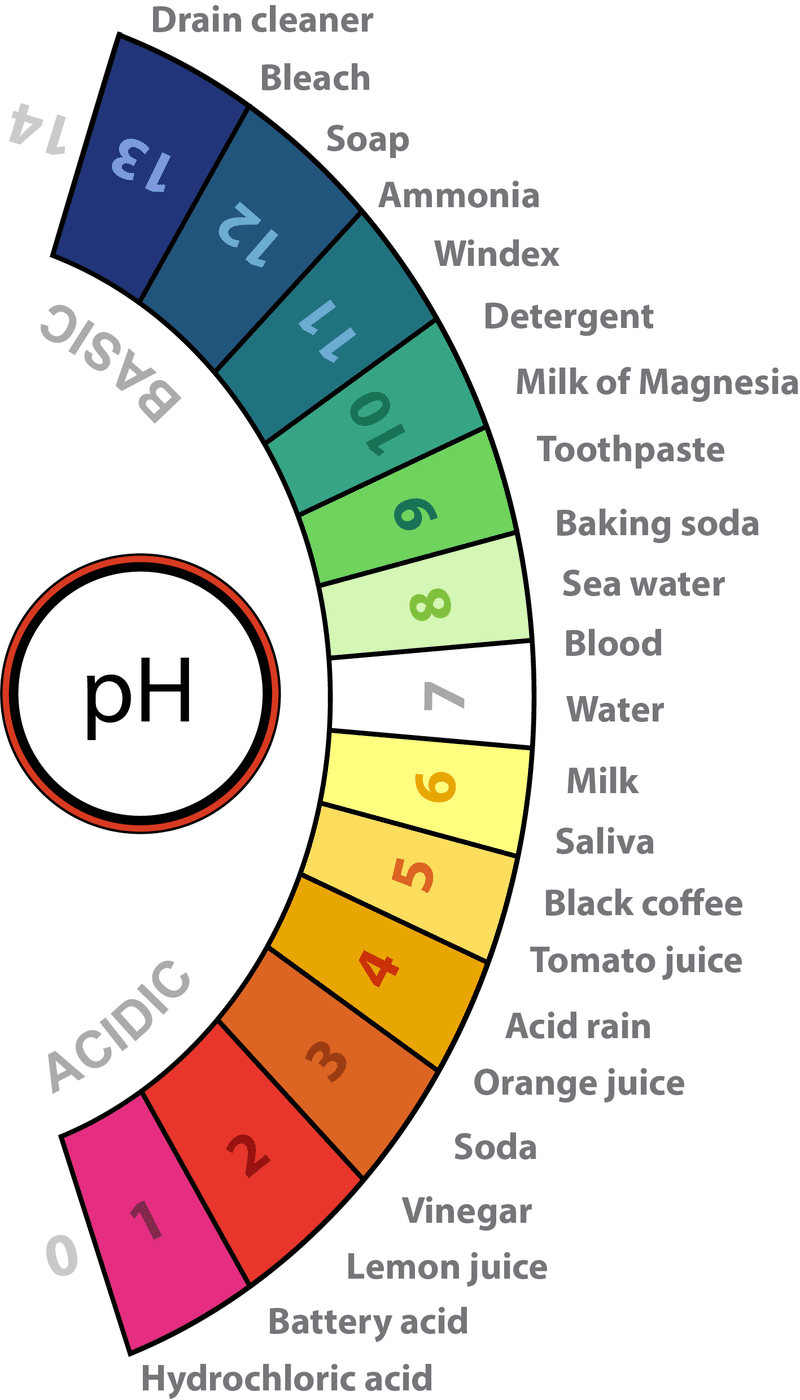
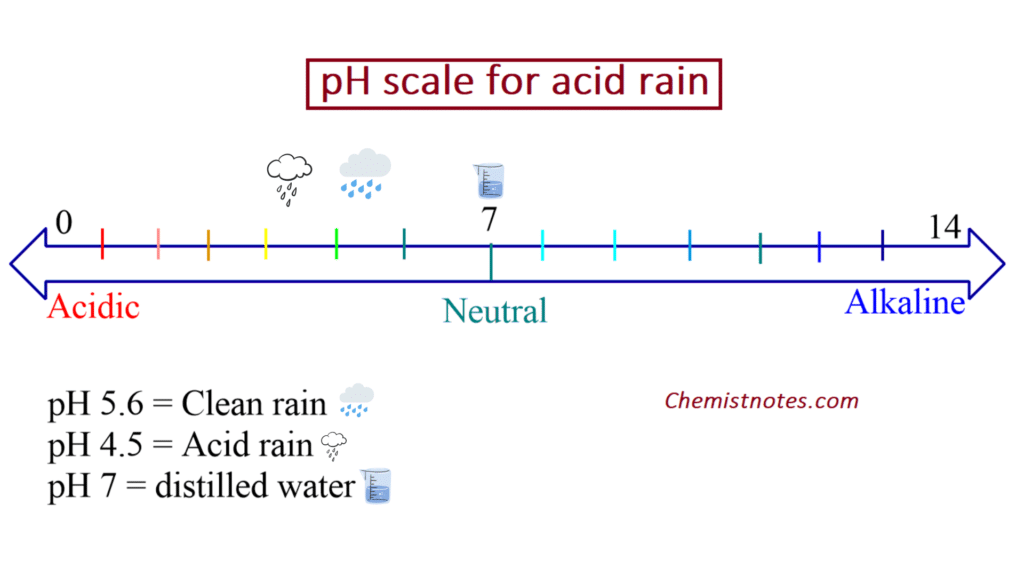
Ph Of Acid Rain Normal clean rain has a pH value of between 5 0 and 5 5 which is slightly acidic However when rain combines with sulfur dioxide or nitrogen oxides produced from power plants and automobiles the rain becomes much more acidic Typical acid rain has a pH value of 4 0
Acid Rain Firstly let us understand exactly what acid rain is The pH level of rainwater is supposed to be 5 6 While the pH value of water is 7 which is what we consider neutral rainwater has a more acidic natu Normal clean rain has a pH value of between 5 0 and 5 5 which is slightly acidic However when rain combines with sulfur dioxide or nitrogen oxides produced from power plants and automobiles the rain becomes much more acidic Typical acid rain has a pH value of 4 0
Ph Of Acid Rain
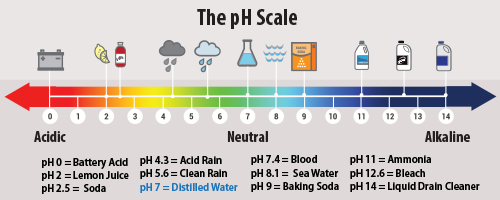
Ph Of Acid Rain
https://www.epa.gov/sites/default/files/styles/medium/public/2016-03/500x350_phscale_3-2.png?itok=QoCEklrC
Water Acids And Bases CK 12 Foundation
https://dr282zn36sxxg.cloudfront.net/datastreams/f-d:92e54d3a10fca553846242aa50101b794cfa094d539447ace167c753%2BIMAGE_TINY%2BIMAGE_TINY.1

PH Of Acid Rain Strong Or Weak Techiescientist
https://techiescientist.com/wp-content/uploads/2021/12/pH-of-Acid-Rain.jpg
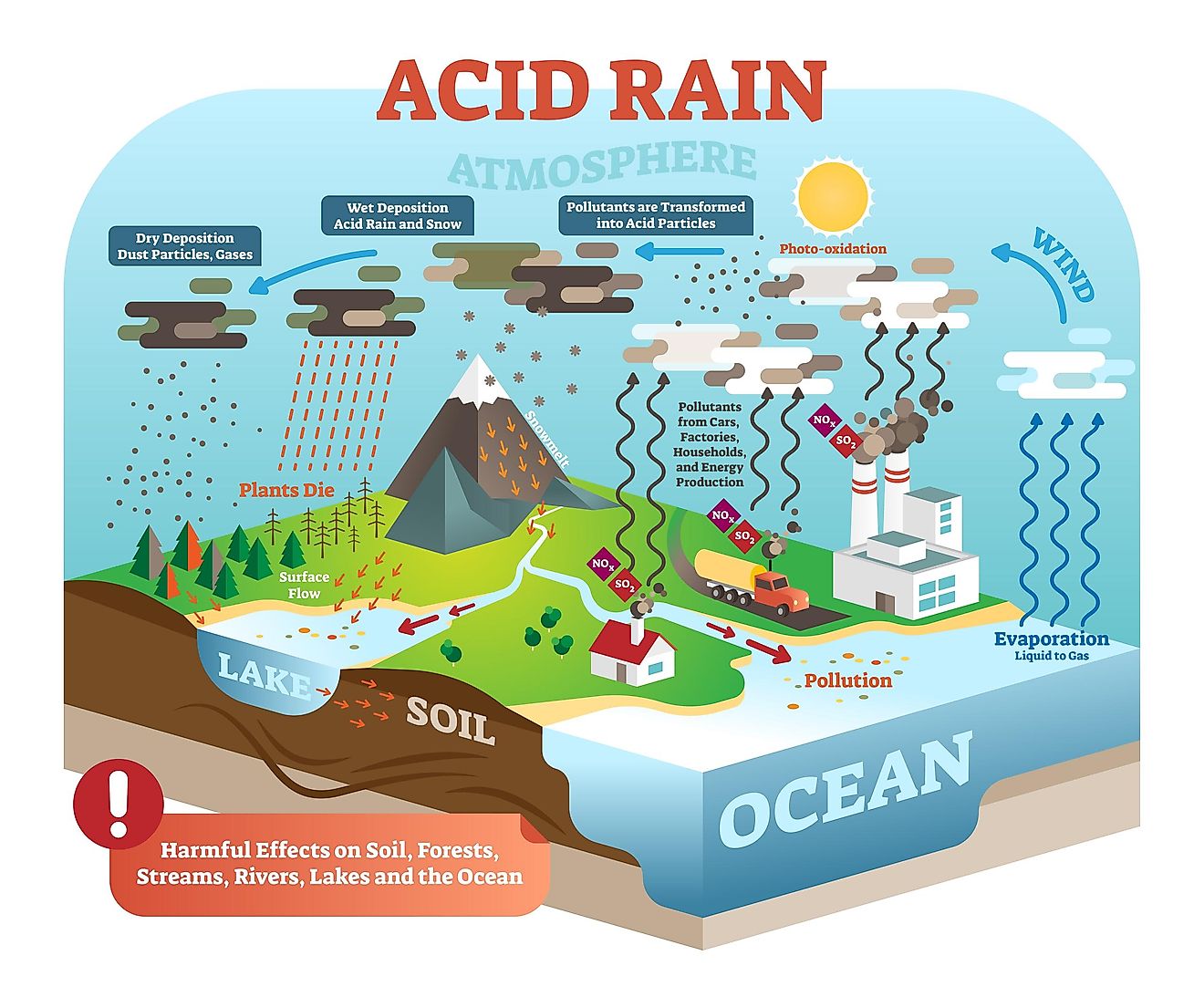
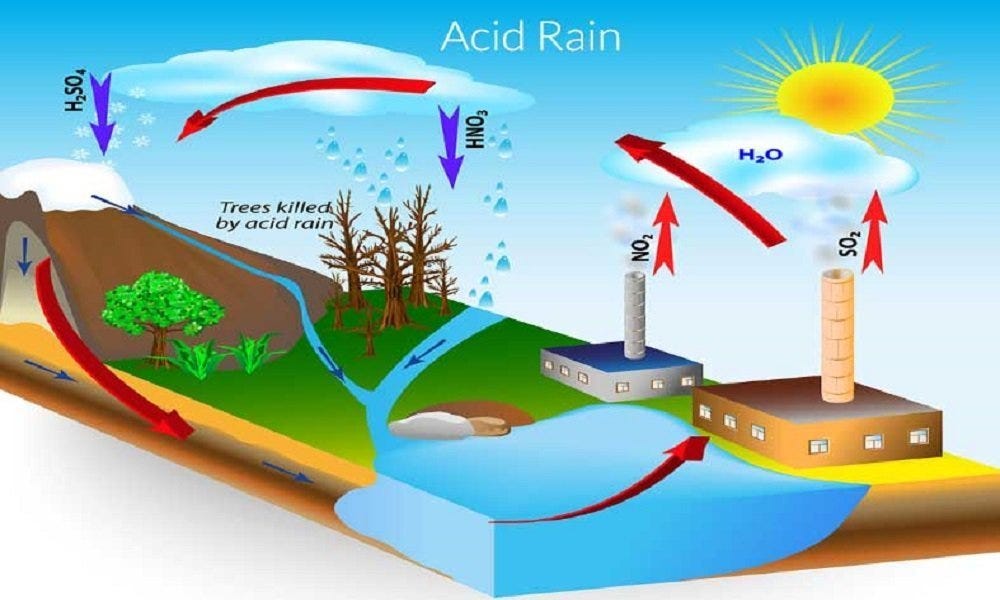
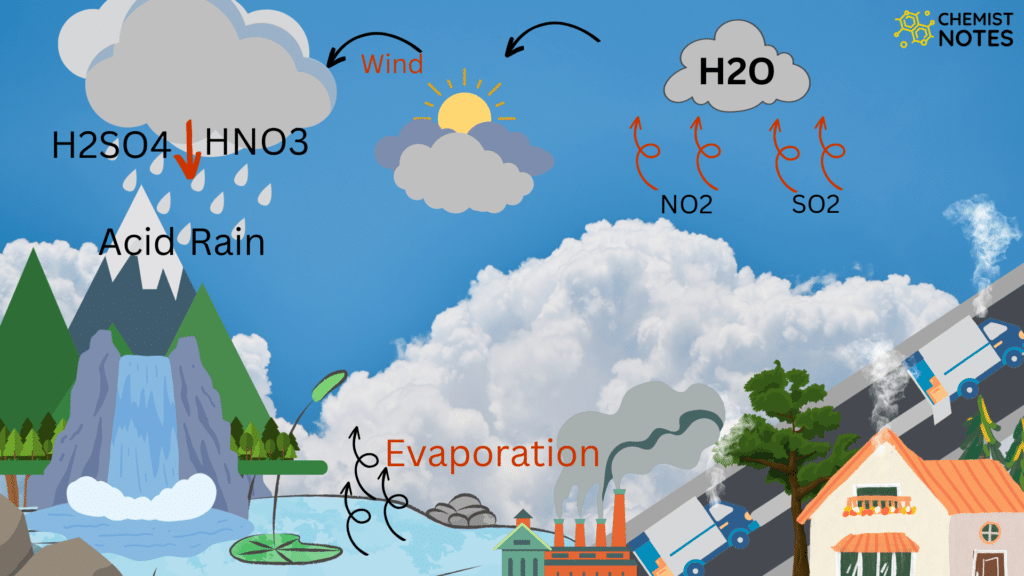
Acid rain is a rain that is acidic meaning it has very low pH When sulfur dioxide and nitrogen oxides are released into the air they react with water and oxygen and produces sulfuric and nitric acids These acids then mix with water and result in acid rain Normal rain water is slightly acidic with a pH range of 5 6 Acid rain involves a major process of acidification of the gases on the interaction with water vapour The main components of acid rain are formed by the reaction of emitted sulfur dioxide and nitrogen oxide with water vapour molecules in the air This reaction of water and oxides result in the formation of acids
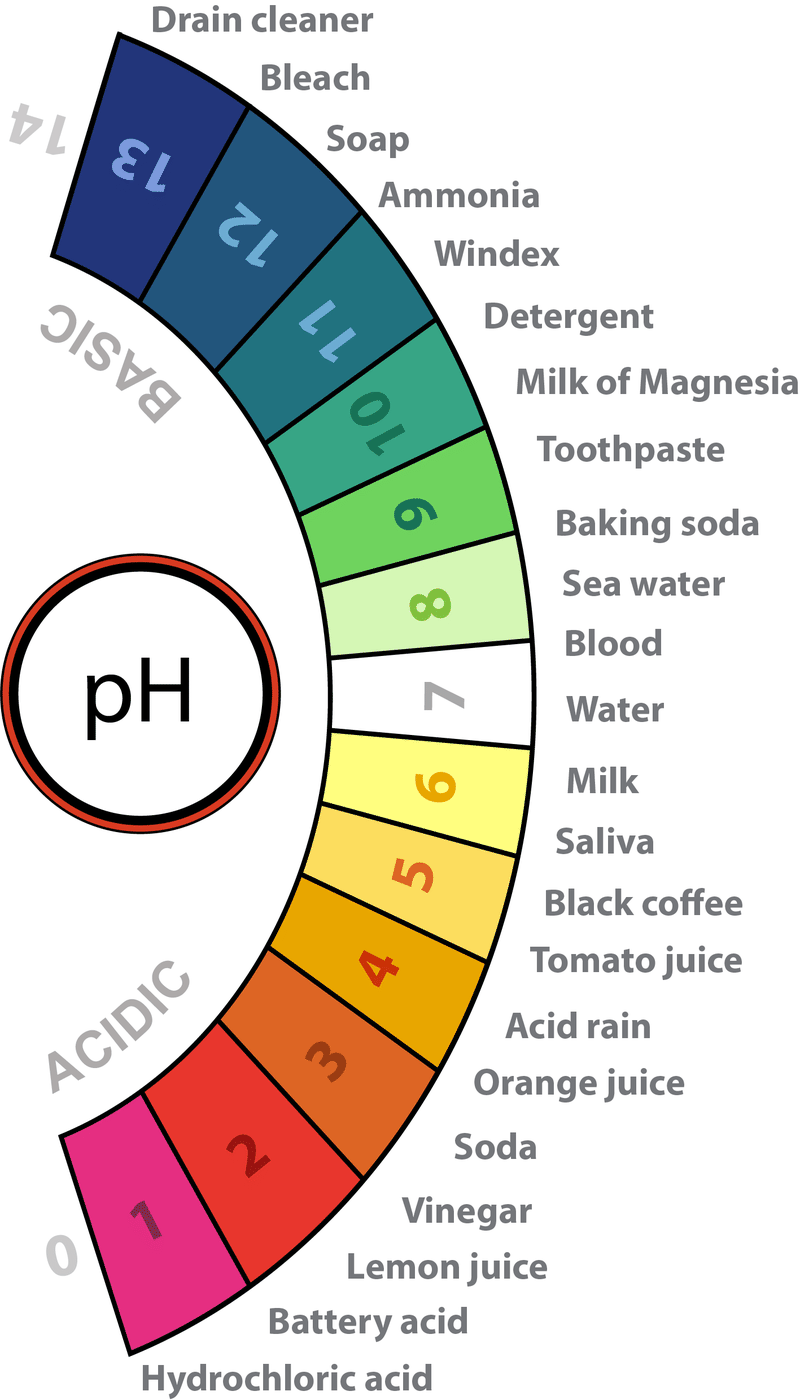
Assertion A The pH of acid rain is less than 5 6 Reason R Carbon dioxide present in the atmosphere dissolves in rain water and forms carbonic acid View Solution The pH of acid rain is less than 5 6 the range is 5 to5 5 Carbon dioxide present in the atmosphere dissolves in rain water and forms carbonic acid Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion
More picture related to Ph Of Acid Rain
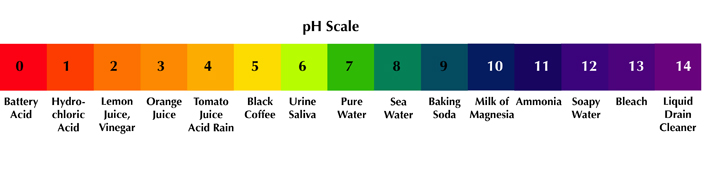
Acid Rain
https://www.exploringnature.org/graphics/Environment/pH_scale.jpg
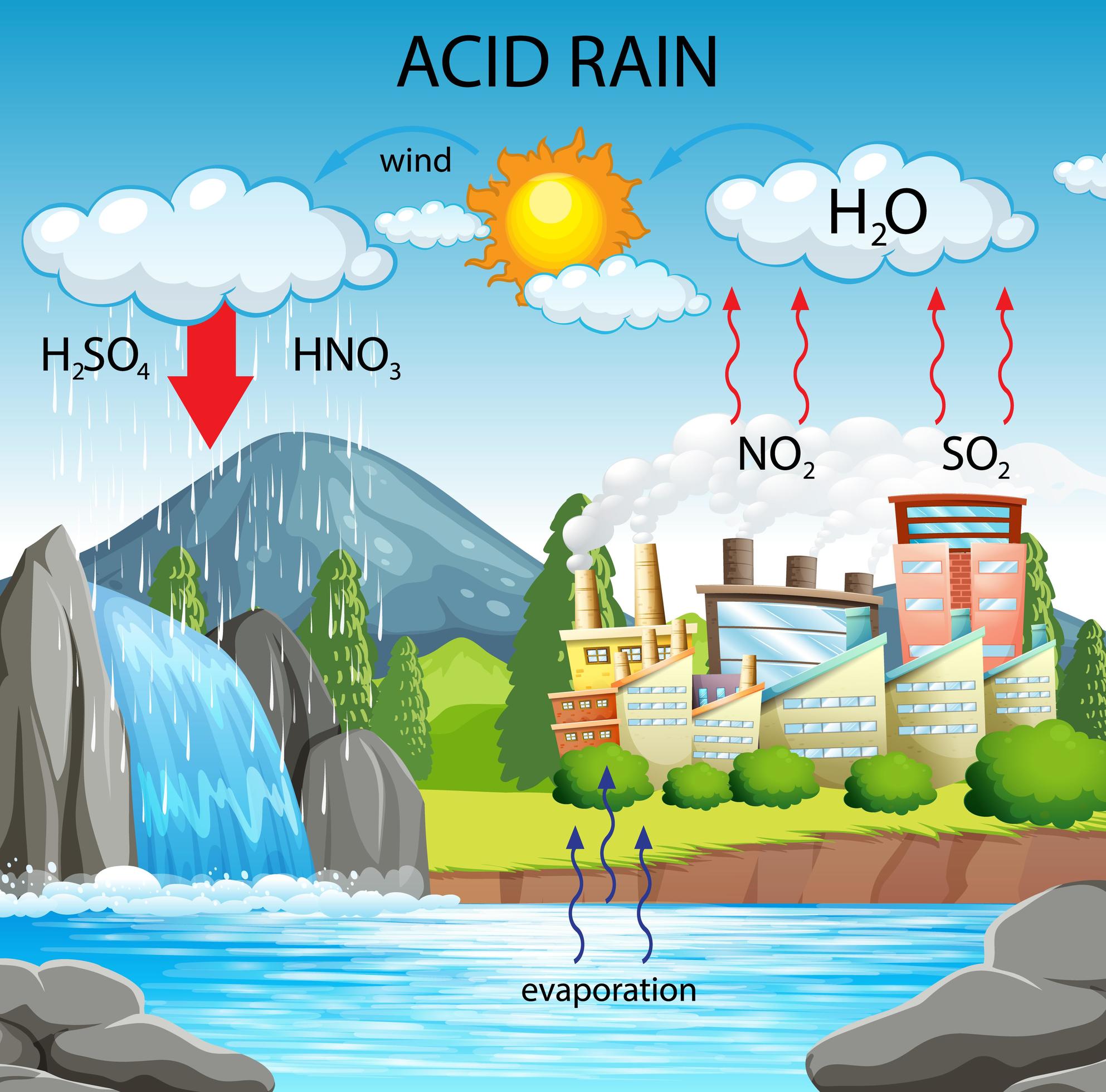
Diagram Showing Acid Rain Pathway 1783983 Vector Art At Vecteezy
https://static.vecteezy.com/system/resources/previews/001/783/983/large_2x/diagram-showing-acid-rain-pathway-free-vector.jpg
Chemistry Of Acid Rain Formation
https://www.worldatlas.com/r/w1200-q80/upload/59/bb/e4/shutterstock-1093338419.jpg
When the pH of the rain water drops below 5 6 it is called acid rain Acid rain refers to the ways in which acid from atmosphere is deposited on earth s surface The deposition of acid takes place in two ways wet and dry ii Acid rain can make the survival of aquatic animals difficult because it can lower the pH of water bodies When the pH of water decreases due to acid rain it becomes more acidic This acidity can harm aquatic life by disrupting their internal pH balance affecting their respiratory systems and damaging their habitats
[desc-10] [desc-11]
Acid Rain Chemistry Notes
https://chemistnotes.com/wp-content/uploads/2023/03/Untitled-design-1024x576.png

Acids Bases And The Ph Scale Worksheets
https://i.pinimg.com/originals/fc/b6/e8/fcb6e89012b2757b43b87040870f6648.jpg

https://www.toppr.com › ask › question › what-is-the-ph-of-acid-rain
Normal clean rain has a pH value of between 5 0 and 5 5 which is slightly acidic However when rain combines with sulfur dioxide or nitrogen oxides produced from power plants and automobiles the rain becomes much more acidic Typical acid rain has a pH value of 4 0
https://www.toppr.com › guides › chemistry › environmental-chemistry › …
Acid Rain Firstly let us understand exactly what acid rain is The pH level of rainwater is supposed to be 5 6 While the pH value of water is 7 which is what we consider neutral rainwater has a more acidic natu
Acid Rain Biology Experts Notes Medium

Acid Rain Chemistry Notes

Acid Rain Pollution Effects Solutions Britannica
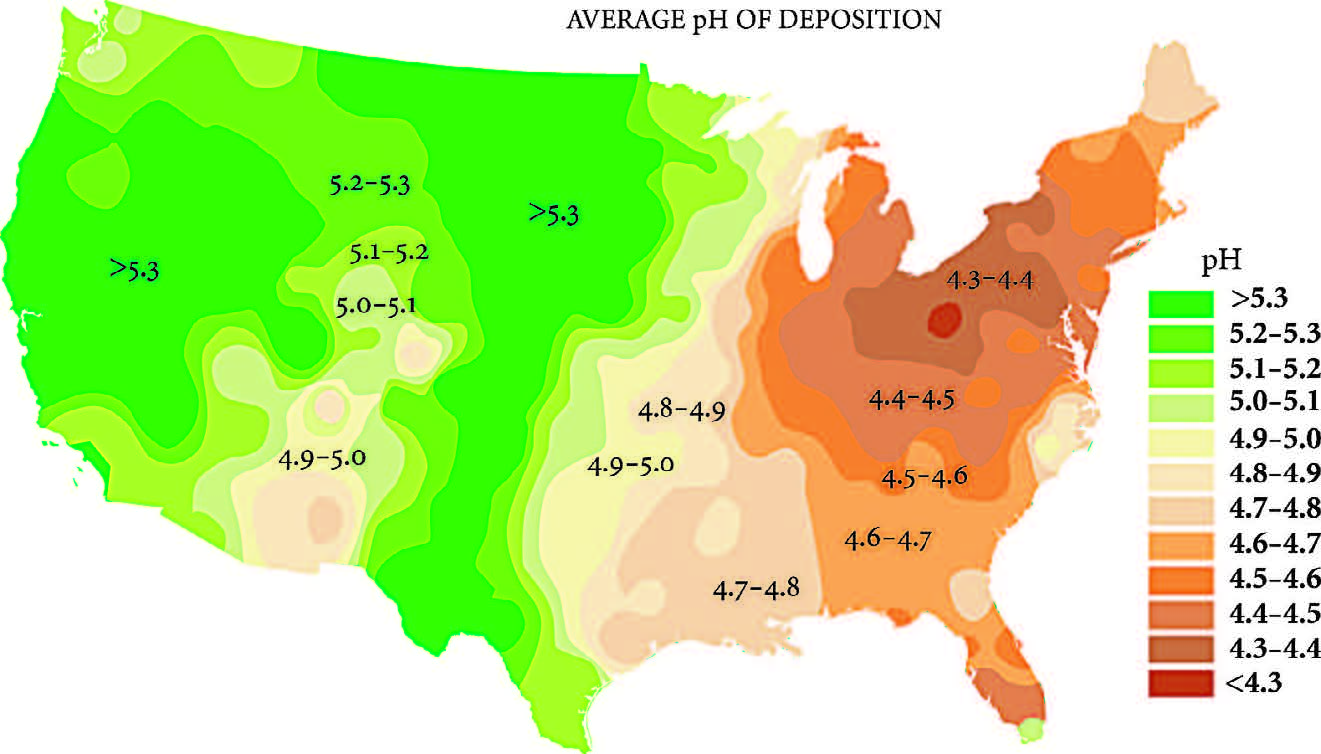
ADK Forever Wild Rock On Adirondacks
Acid Rain Chemistry Notes
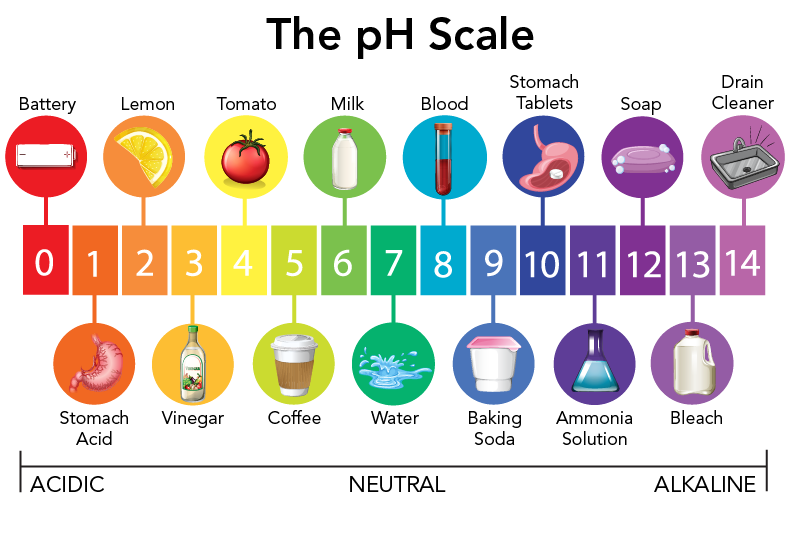
Explain The Ph Difference Between Acid Rain And Pure Water

Explain The Ph Difference Between Acid Rain And Pure Water
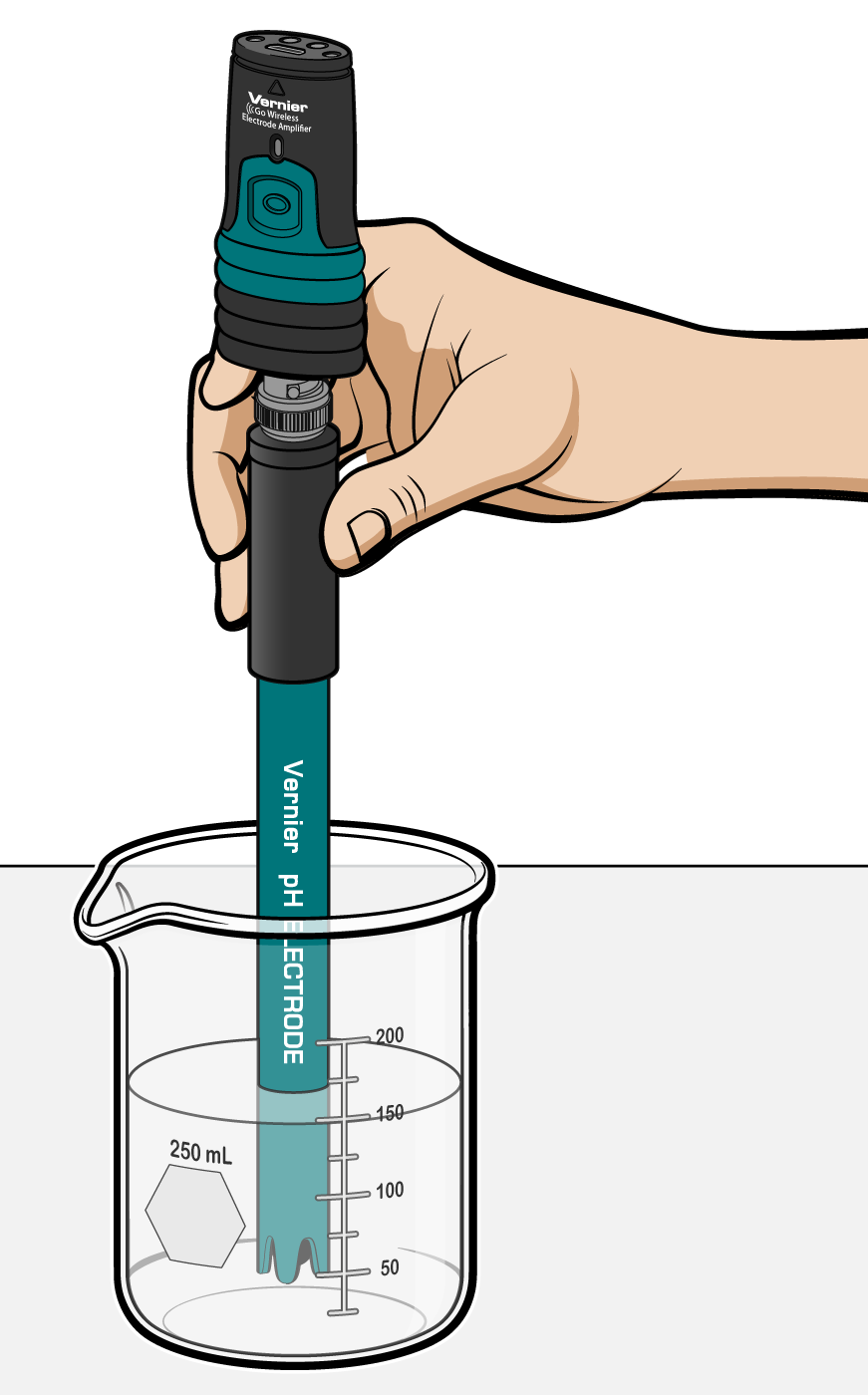
Soil And Acid Rain Experiment 8 From Earth Science With Vernier
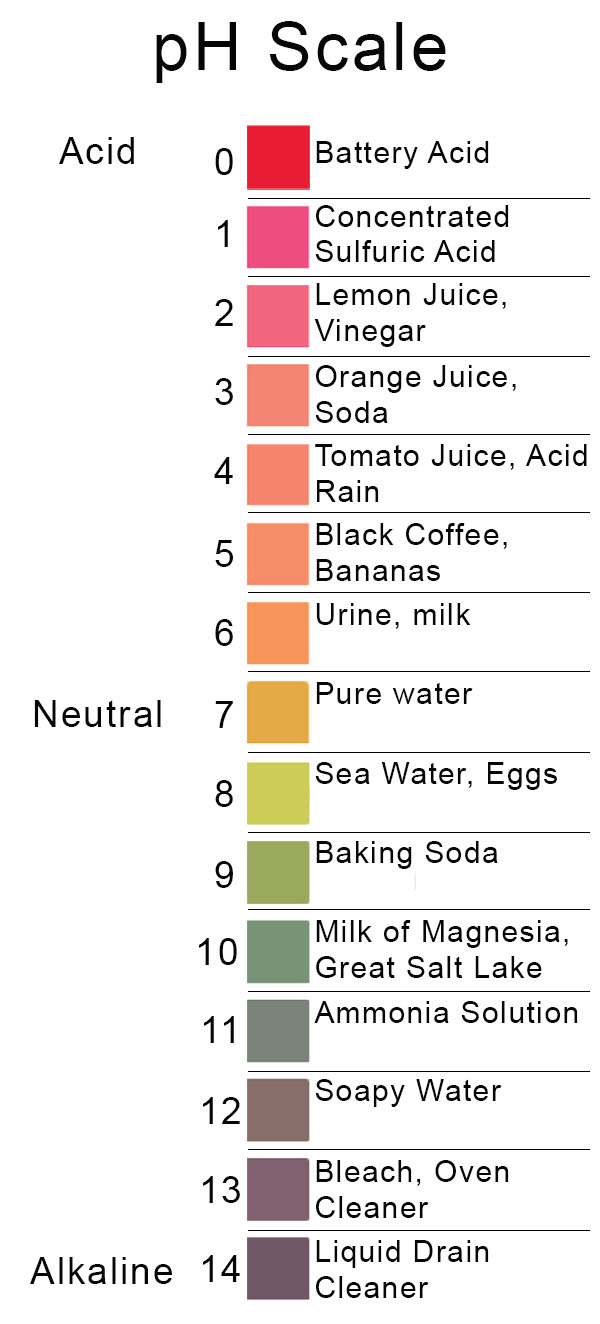
Ph Level Of Orange Juice
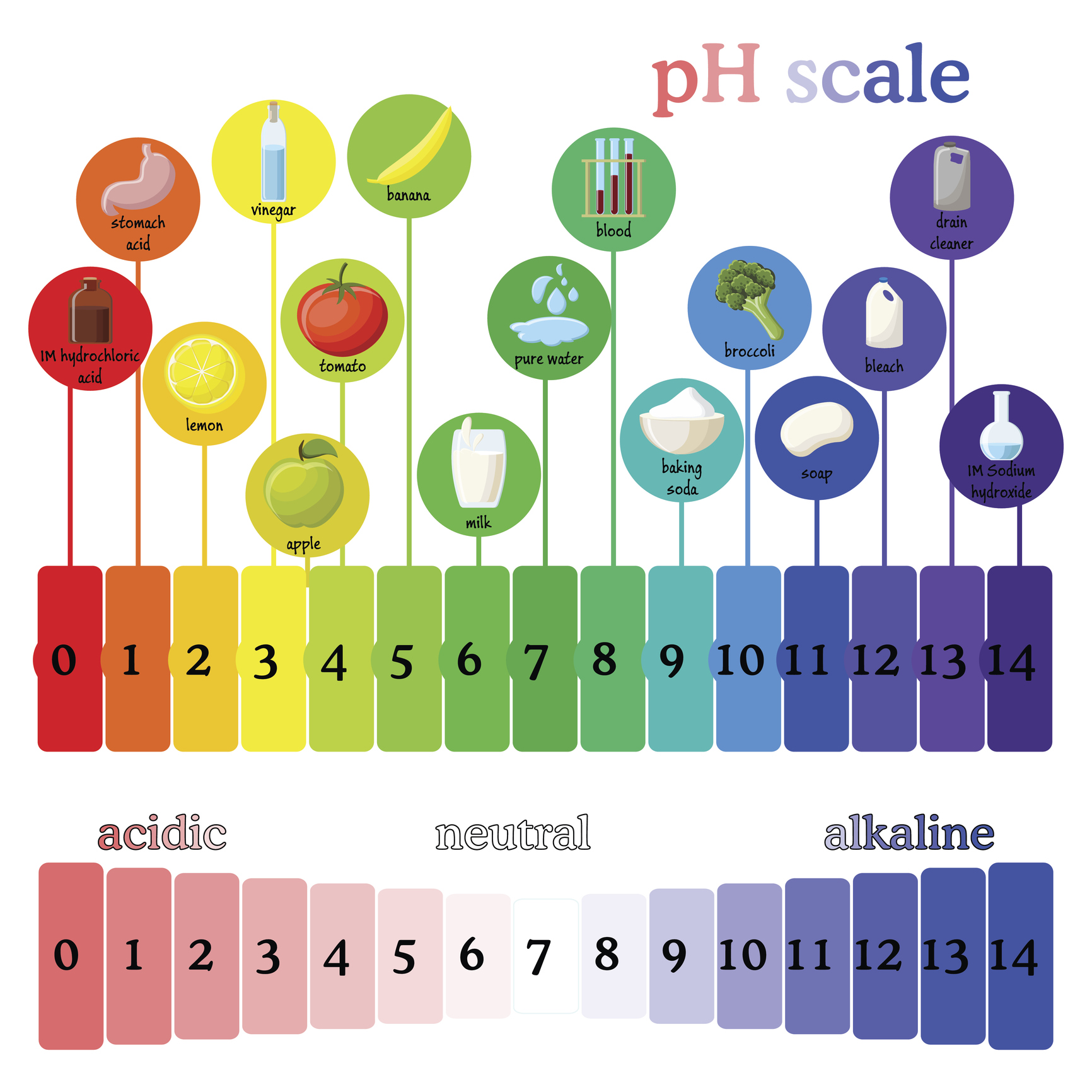
How To Test For PH Archives Musto Wine Grape Company LLC
Ph Of Acid Rain - Acid rain is a rain that is acidic meaning it has very low pH When sulfur dioxide and nitrogen oxides are released into the air they react with water and oxygen and produces sulfuric and nitric acids These acids then mix with water and result in acid rain Normal rain water is slightly acidic with a pH range of 5 6