What Is Metallic Bonding What is Metallic Bonding A metallic bond is a type of chemical bond in which a cloud of free moving valence electrons is bonded to the positively charged ions in a metal It can be described as the sharing of free electrons among a lattice of positively charged metal ions
What is a Metallic Bond Metallic bond is a term used to describe the collective sharing of a sea of valence electrons between several positively charged metal ions A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations In contrast covalent and ionic bonds form between two discrete atoms
What Is Metallic Bonding
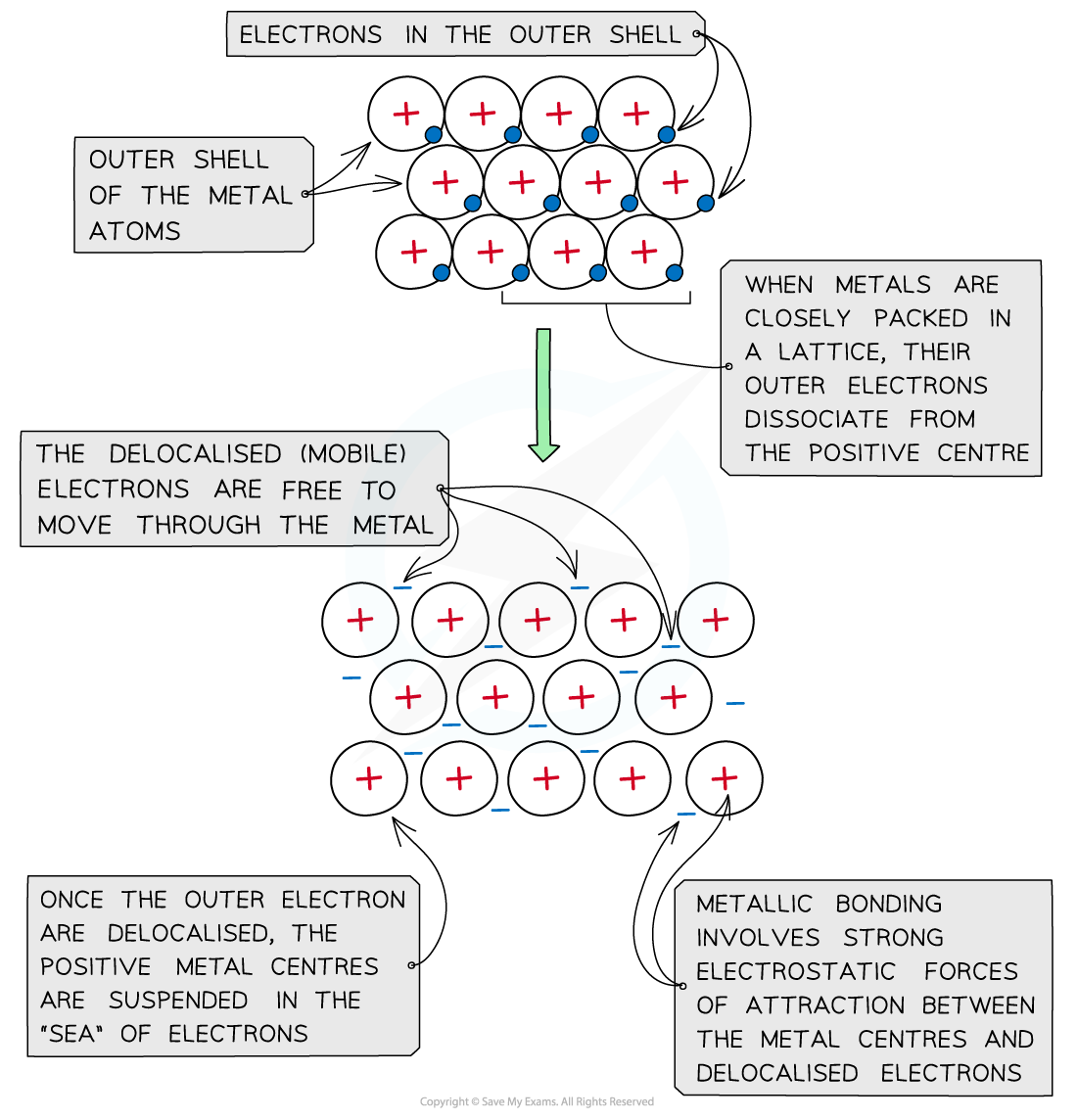
What Is Metallic Bonding
https://oss.linstitute.net/wechatimg/2022/08/1.3-Chemical-Bonding-Diagram-to-show-metallic-bonding-3.png

Online Essay Help Amazonia fiocruz br
https://cdn.britannica.com/21/2621-050-B04050DD/bonding-solids.jpg

What Is A Metallic Bond And How Does It Form Metallic Bonding
https://i.ytimg.com/vi/9TAkUkrphdg/maxresdefault.jpg
In metallic bonding metal atom nuclei share delocalized valence electrons Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons These free electrons are called delocalized because they are not confined localized to one atom Metallic bonding is the force of attraction between valence electrons and the metal atoms It is the sharing of many detached electrons between many positive ions where the electrons act as a glue giving the substance a definite structure It is unlike covalent or ionic bonding
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons in the form of an electron cloud of delocalized electrons and positively charged metal ions In the outer shells of the metal atoms are free to move The metallic bond is the force of attraction between these free moving delocalised electrons and positive metal
More picture related to What Is Metallic Bonding
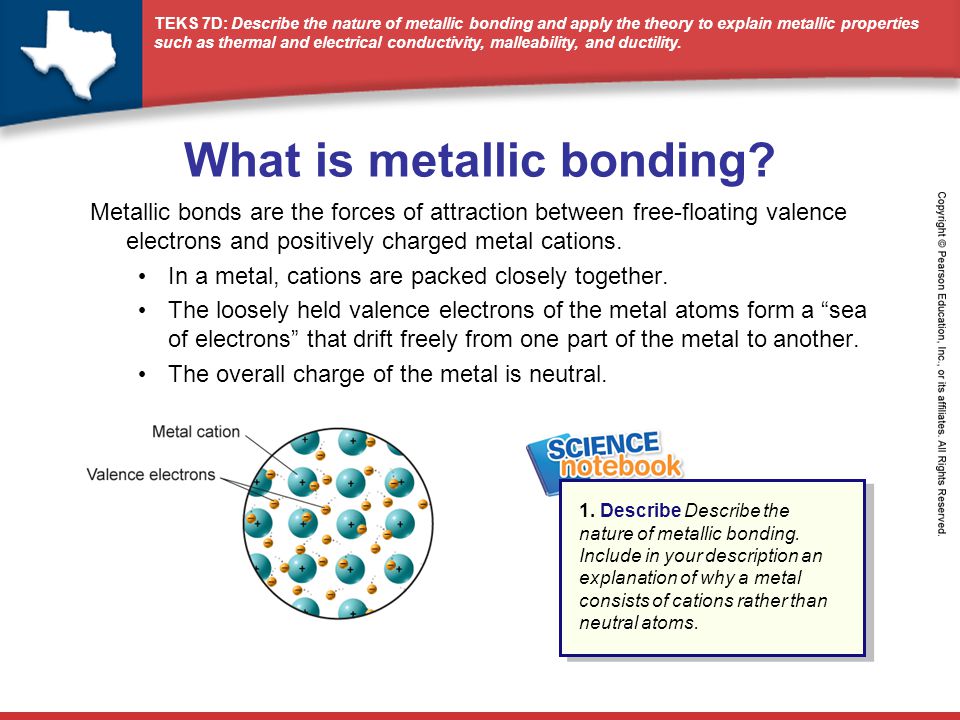
What Is A Metallic Bond
https://slideplayer.com/4890517/16/images/slide_1.jpg

Metallic Bond Definition Examples And Diagrams
https://www.chemistrylearner.com/wp-content/uploads/2020/09/Metallic-Bond-Examples.jpg
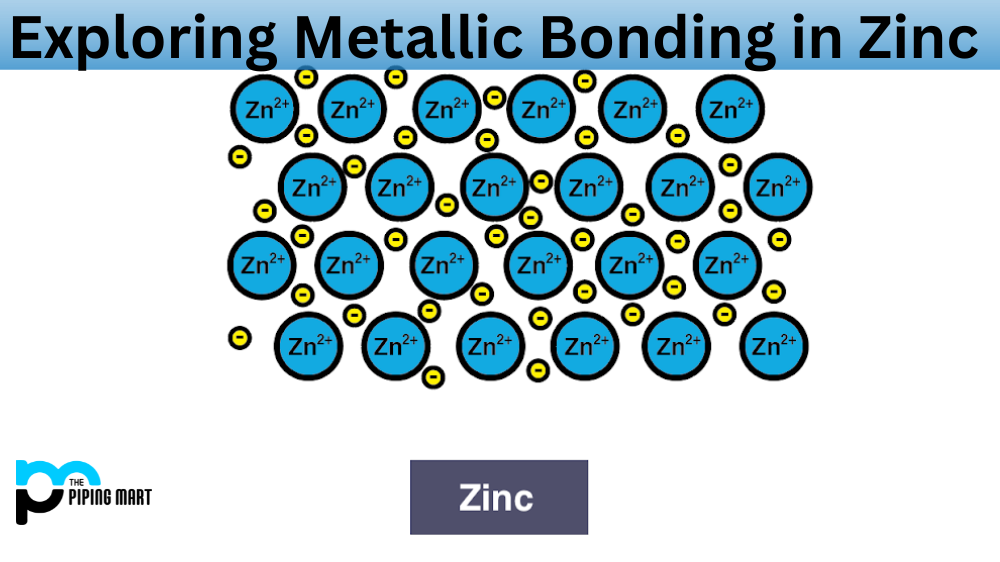
Metallic Bonding In Zinc An Overview
https://cdn.thepipingmart.com/wp-content/uploads/2023/01/Sheet-Metal-Plating-A-Comprehensive-Guide-to-Zinc-Plating-3.png
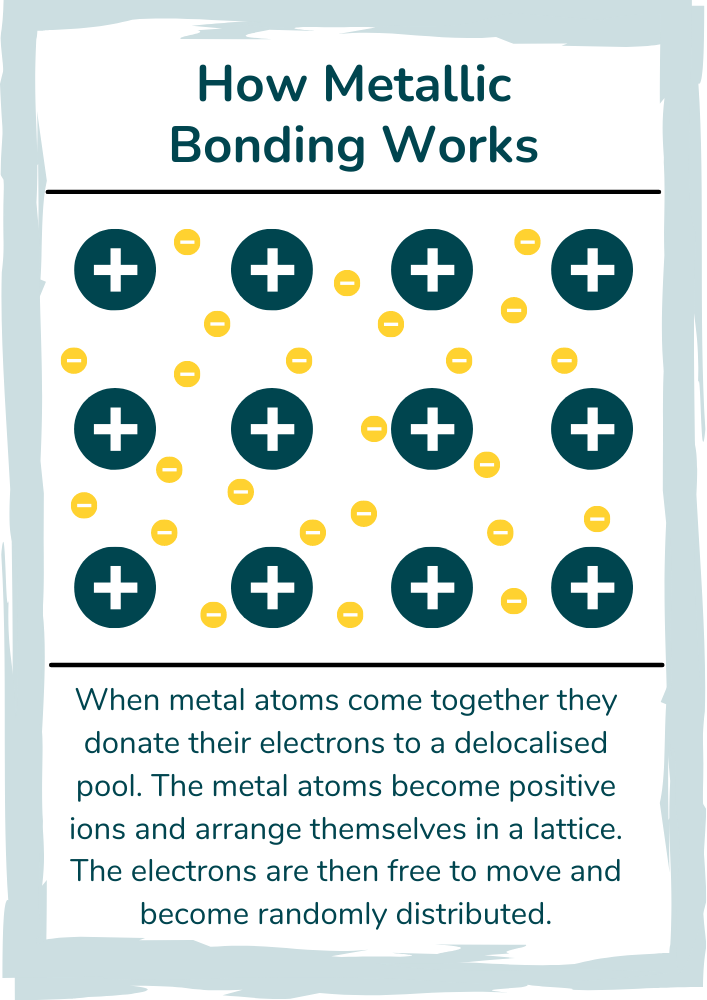
What is Metallic Bonding Metallic bonding is a chemical bonding that occurs as a result of the attraction of delocalized electrons and positive ions Metal is made up of its delocalized electrons and numerous positive metal ions arranged in a regular pattern packed closely together In chemistry metallic bonding is a type of chemical bonding that occurs between metal atoms It is responsible for the unique properties of metals such as their high electrical and thermal conductivity ductility and malleability
[desc-10] [desc-11]
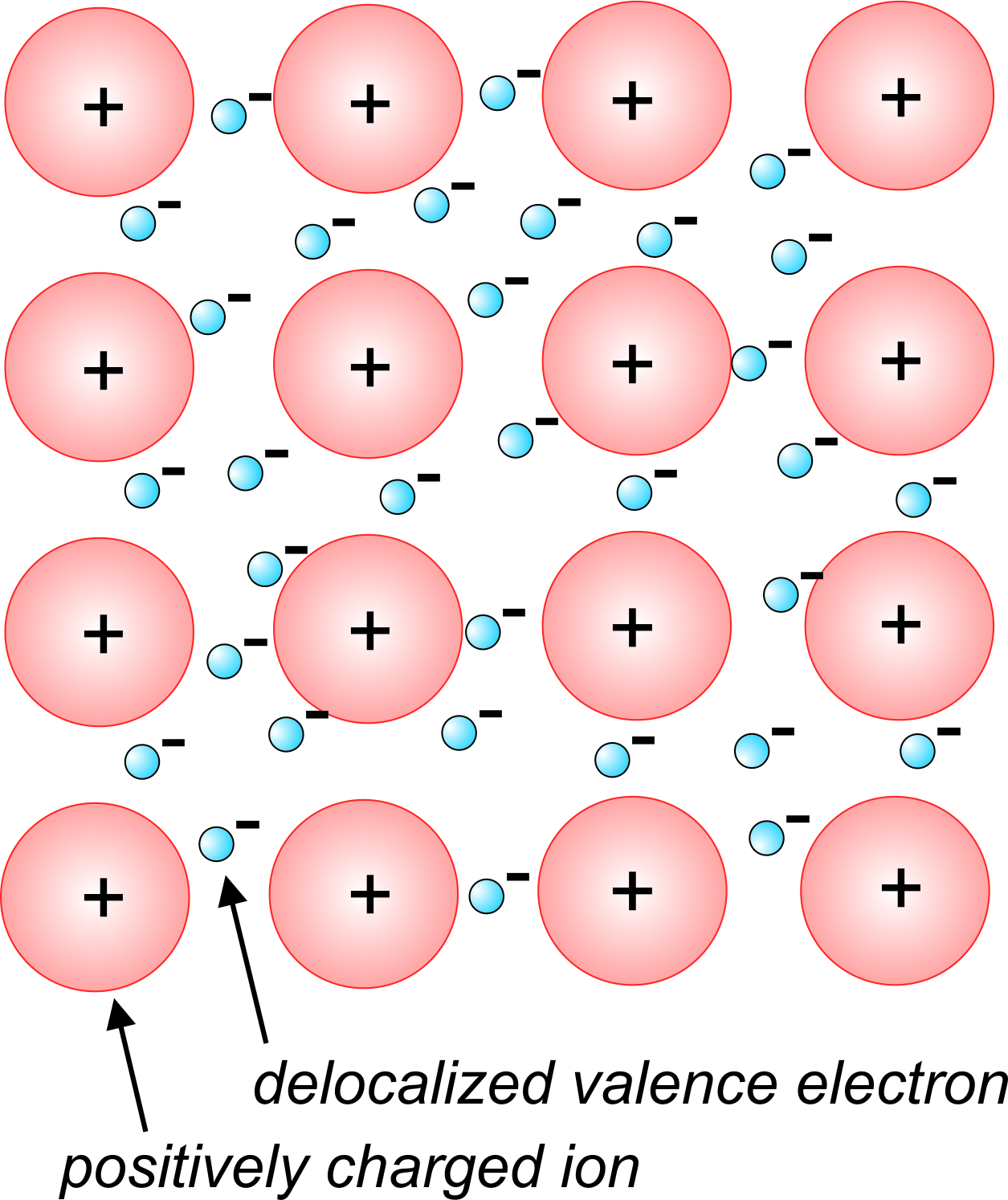
2 4 3 Metallic Bonds Geosciences LibreTexts
https://opengeology.org/Mineralogy/wp-content/uploads/2020/01/metallic-bonding-v2.jpg

What Is Metallic Bonding
https://i.pinimg.com/originals/db/bc/a4/dbbca415452d2b465540e12c63a3b251.png
https://www.chemistrylearner.com/chemical-bonds/metallic-bond
What is Metallic Bonding A metallic bond is a type of chemical bond in which a cloud of free moving valence electrons is bonded to the positively charged ions in a metal It can be described as the sharing of free electrons among a lattice of positively charged metal ions

https://byjus.com/chemistry/metallic-bonds
What is a Metallic Bond Metallic bond is a term used to describe the collective sharing of a sea of valence electrons between several positively charged metal ions

Isaac Die Absicht Notwendig An Example Of A Metallic Bond Mit

2 4 3 Metallic Bonds Geosciences LibreTexts
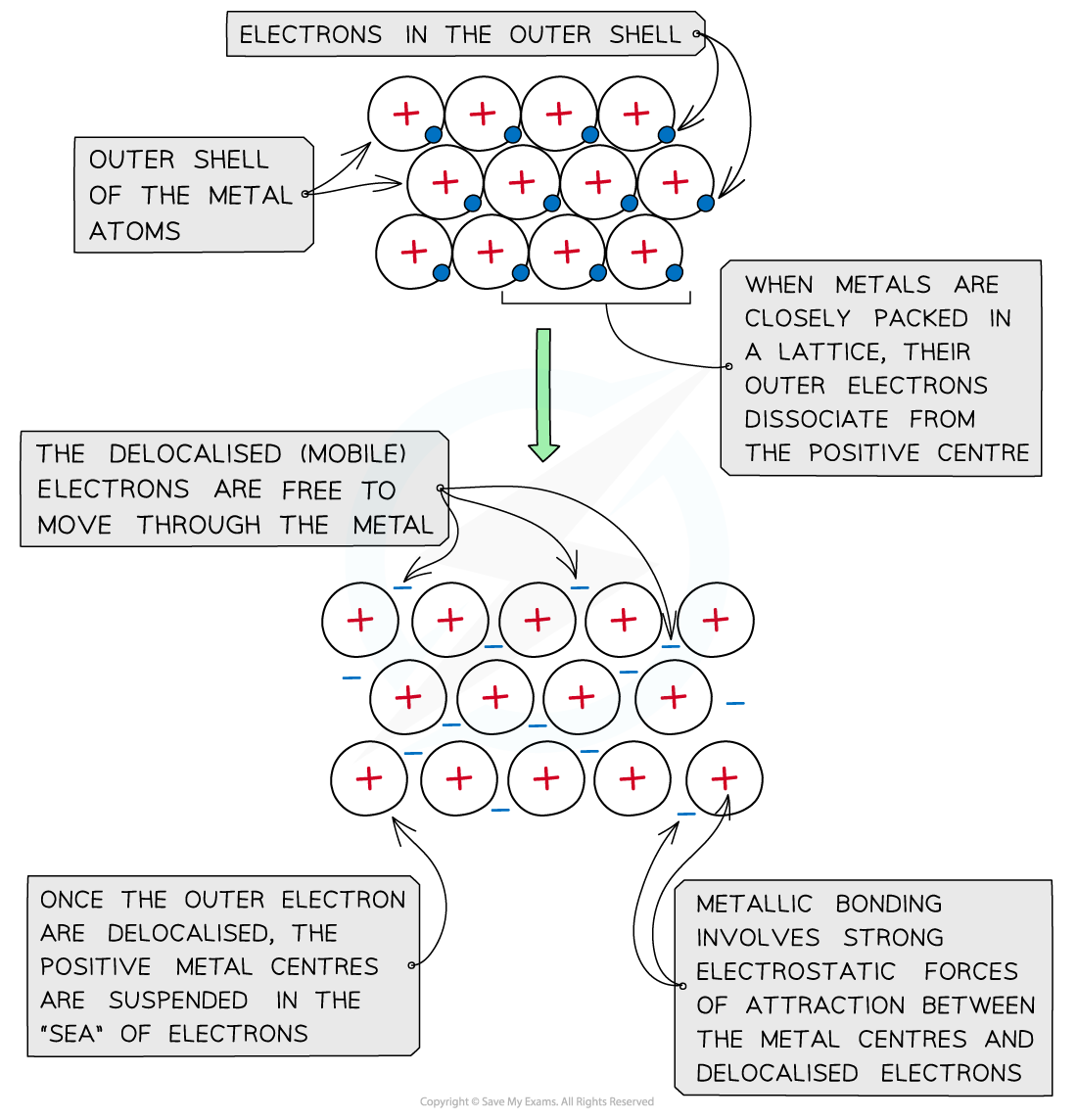
Metallic Bonding Explained Discover Tutoring

Ductility Meaning Definition Comparison Of Malleability And

Metallic Bonding Chemistry Definition DEFINITION GHW
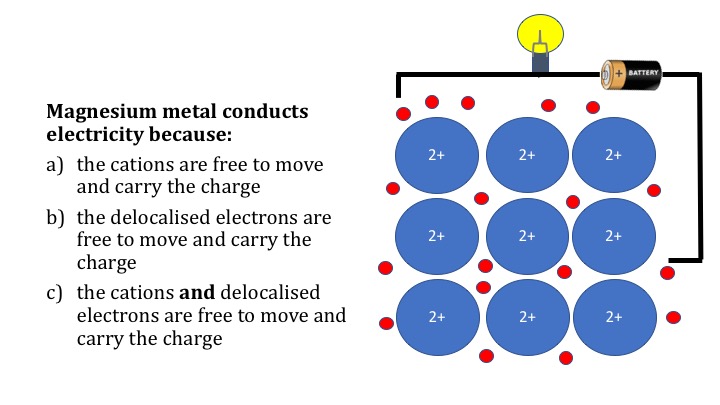
Metallic Bonding Teaching Resources The Science Teacher

Metallic Bonding Teaching Resources The Science Teacher

Metallic Bonding Review Jeopardy Template
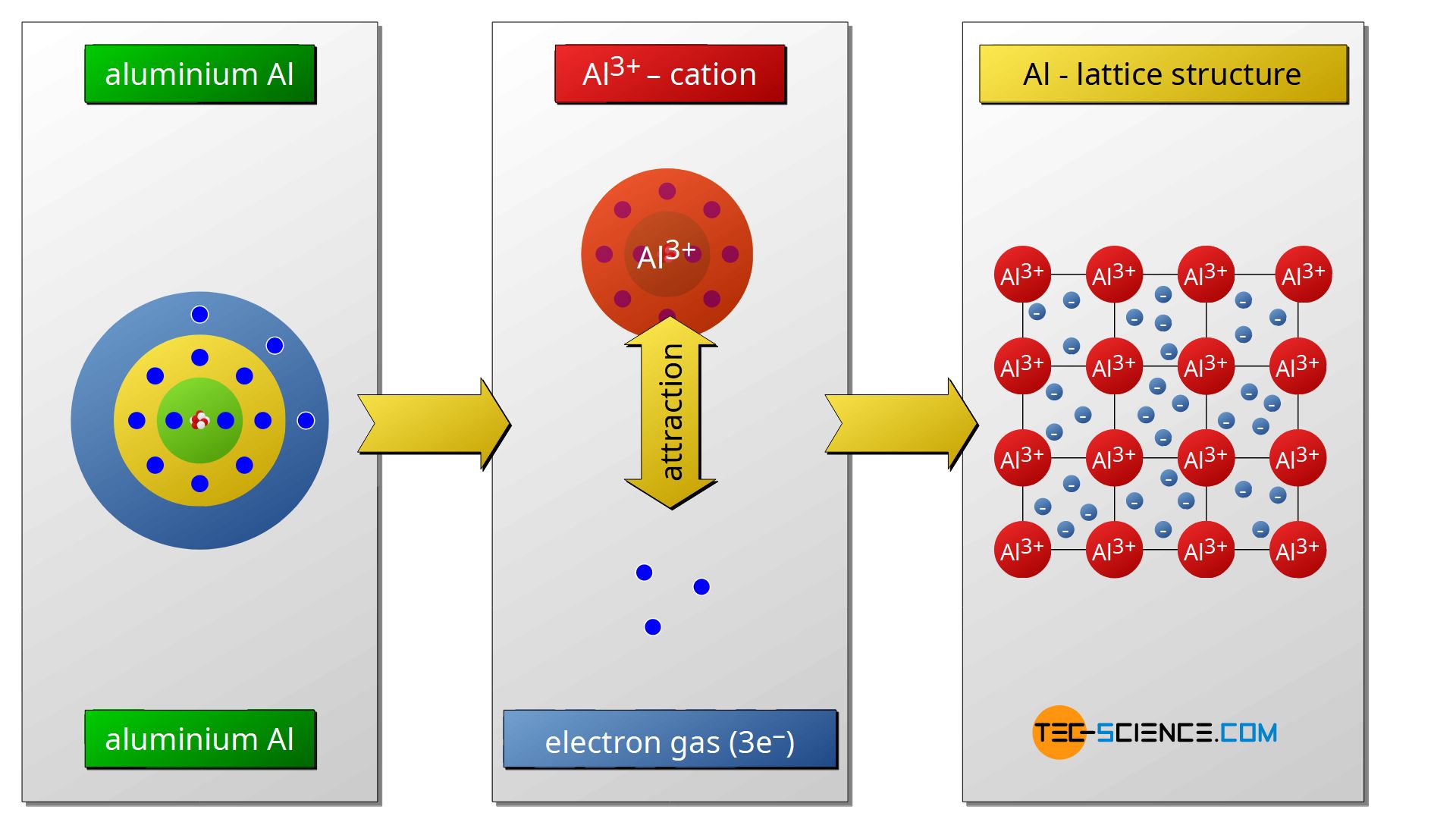
Metallic Bonding Tec science

Forms Of Binding In Crystals Overall Science
What Is Metallic Bonding - In the outer shells of the metal atoms are free to move The metallic bond is the force of attraction between these free moving delocalised electrons and positive metal