What Makes Thermal Energy Kinetic Kinetic energy is the total energy of a particle or system For example a ball falling has kinetic energy Thermal energy is a form of kinetic energy inside particles are at least
And how exactly is the kinetic energy turned to thermal energy Simple the unified downward motion of all the particles is turned to random motions by colliding with the earth So why can t The system starts with kinetic energy which goes away replaced with thermal energy This happens by means of work done by kinetic friction
What Makes Thermal Energy Kinetic

What Makes Thermal Energy Kinetic
https://i.pinimg.com/736x/97/94/85/9794858574a2a842070929a39dc28a39.jpg

Kinetic Energy Temperature And Particle Speeds YouTube
https://i.ytimg.com/vi/Lc5TgTdNfzM/maxresdefault.jpg

How To Calculate The Average Translational Kinetic Energy Of Molecules
https://i.ytimg.com/vi/bg5d5BFANVU/maxresdefault.jpg
Vibrational kinetic energy is expressed as an object s vibrational motion e g sound energy rotational kinetic energy occurs when an object rotates or turns e g a pulley Thermal energy comes from the total kinetic energy of all the particles It is measured in joules J A joulemeter is a device that measures heat energy When thermal energy flows we refer to it
Thermal Energy also known as random or internal Kinetic Energy due to the random motion of molecules in a system Kinetic Energy is seen in three forms vibrational Thermal energy also called heat energy is one of many kinds of internal energy in a system Others include potential energy the kinetic energy of translational and rotational
More picture related to What Makes Thermal Energy Kinetic
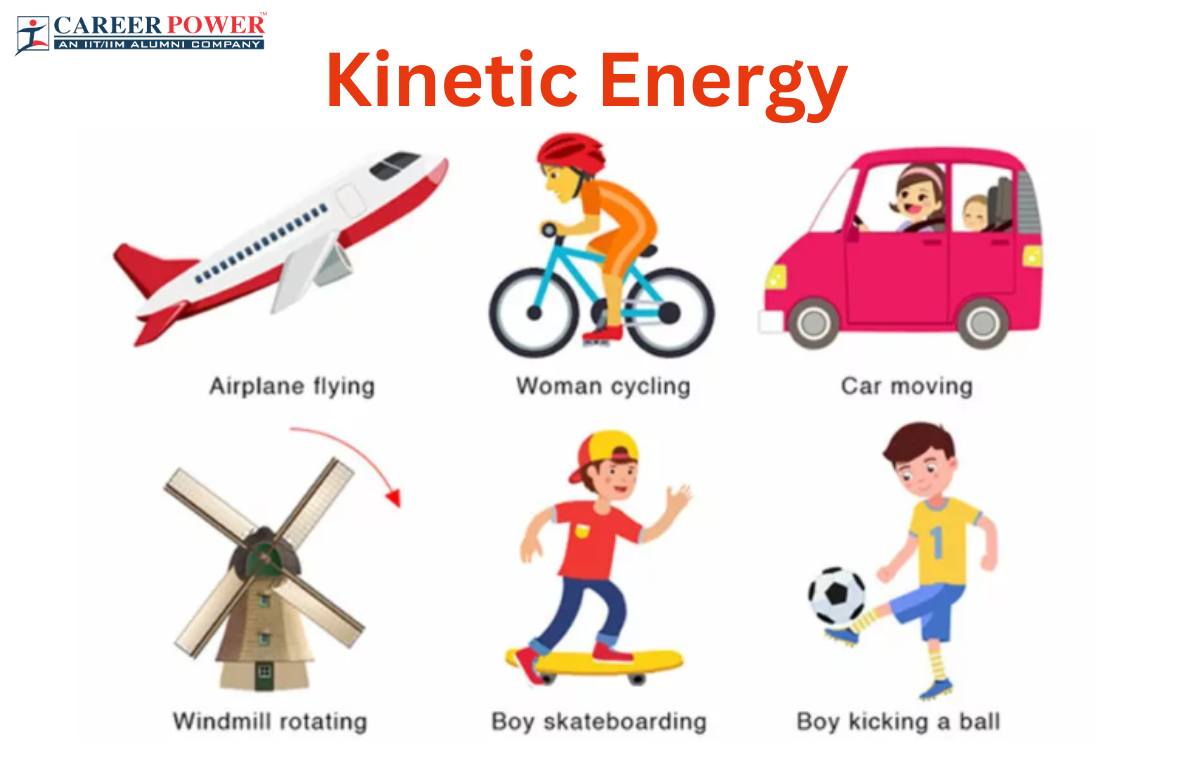
What Is Kinetic Energy Discount Www aikicai
https://www.careerpower.in/blog/wp-content/uploads/sites/2/2023/08/21182701/Kinetic-Energy.png
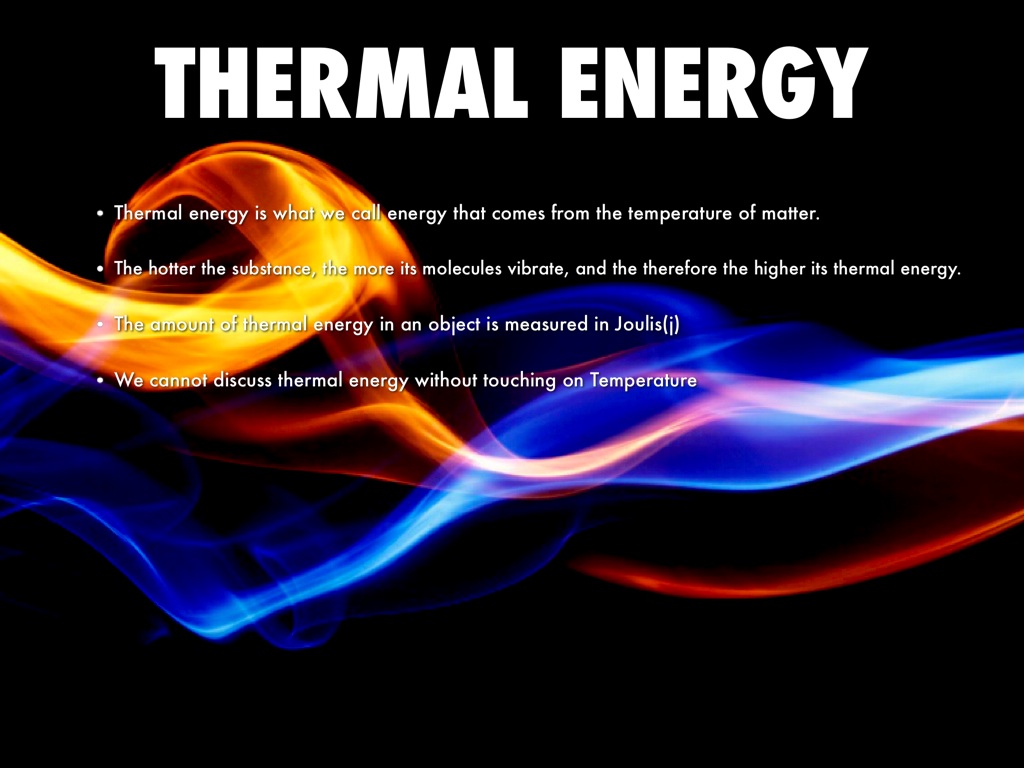
Thermal Energy By Nadia K
https://img.haikudeck.com/mg/E22E231E-ECAF-4303-A8D3-CDBA217A8048.jpg
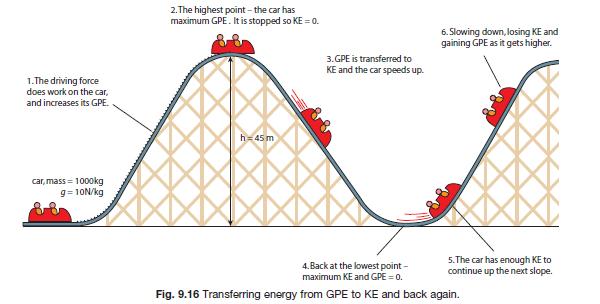
Slide 3
https://revisionworld.com/sites/revisionworld.com/files/imce/transferring energy.jpg
When dealing with the energy of molecules in condensed matter there are two energy terms The first term represents the energy associated with the momentum of the molecule i e the Thermal energy is the internal energy of a substance the sum of the random kinetic energies and potential energies of all the particles atoms that make up the substance Heat is not necessarily the same as thermal energy
The kinetic temperature is the variable needed for subjects like heat transfer because it is the translational kinetic energy which leads to energy transfer from a hot area larger kinetic Thermal energy comes from a substance whose molecules and atoms are vibrating faster due to a rise in temperature Heat energy is another name for thermal energy Kinetic energy is the
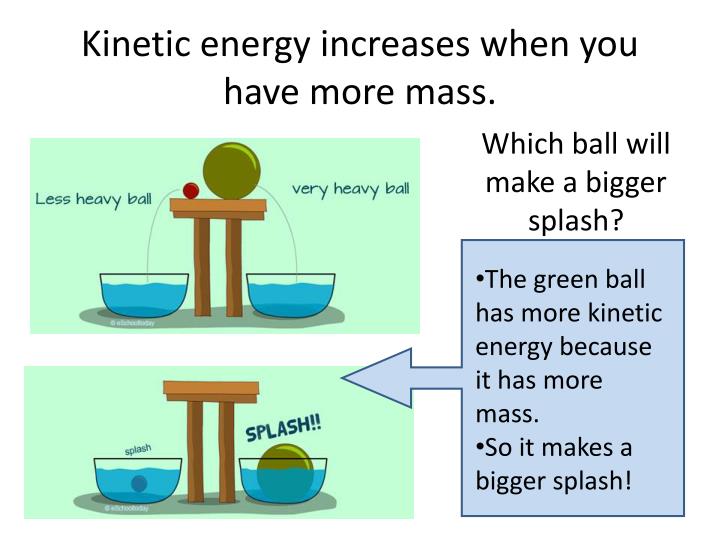
PPT Energy PowerPoint Presentation ID 5798570
https://image3.slideserve.com/5798570/kinetic-energy-increases-when-you-have-more-mass-n.jpg
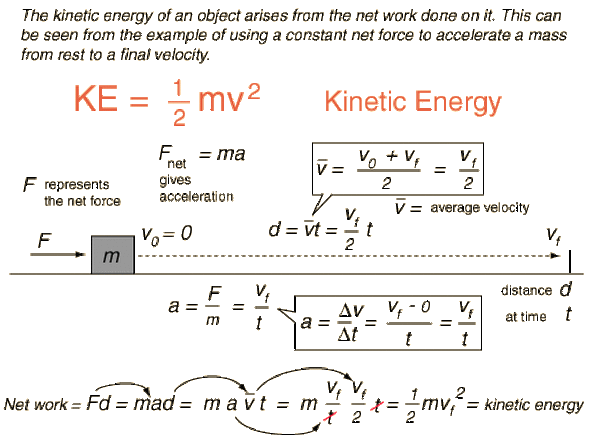
Kinetic Energy Forms Of Energy
http://superawsomeformsofenergy.weebly.com/uploads/2/0/5/9/20594352/3637681.gif?590

https://physics.stackexchange.com › questions › ...
Kinetic energy is the total energy of a particle or system For example a ball falling has kinetic energy Thermal energy is a form of kinetic energy inside particles are at least

https://van.physics.illinois.edu › ask › listing
And how exactly is the kinetic energy turned to thermal energy Simple the unified downward motion of all the particles is turned to random motions by colliding with the earth So why can t

Create A Poster That Illustrates How A Change In Thermal Energy Affects

PPT Energy PowerPoint Presentation ID 5798570

Kinetic Energy Equation Examples Tessshebaylo
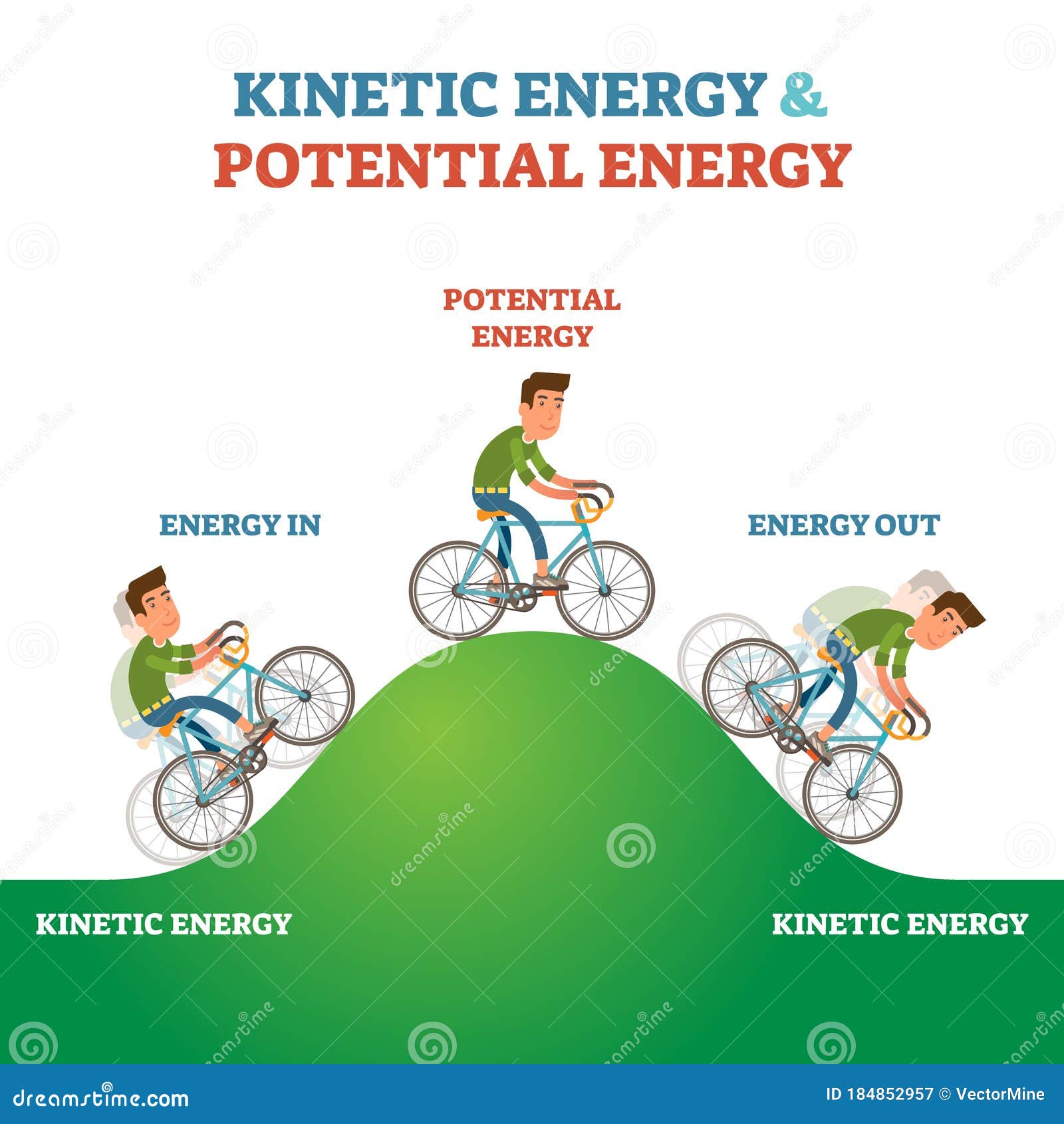
Potential And Kinetic Energy Diagram Vector Illustration
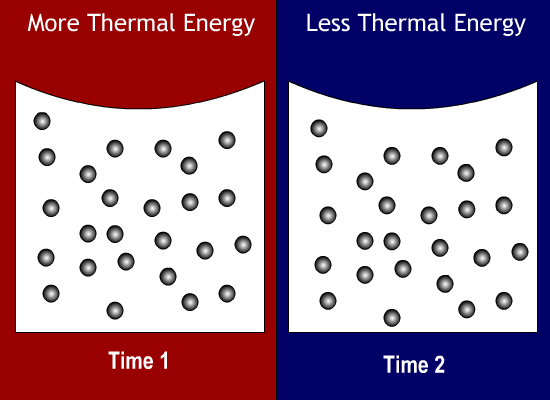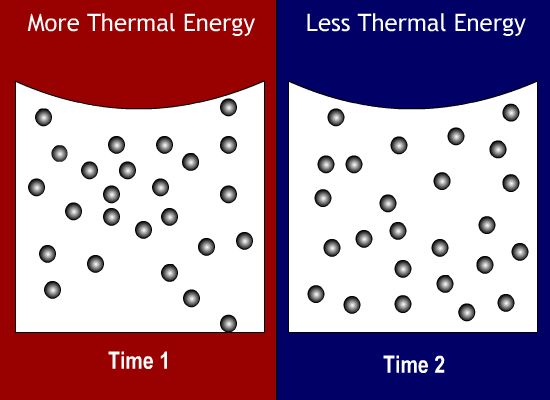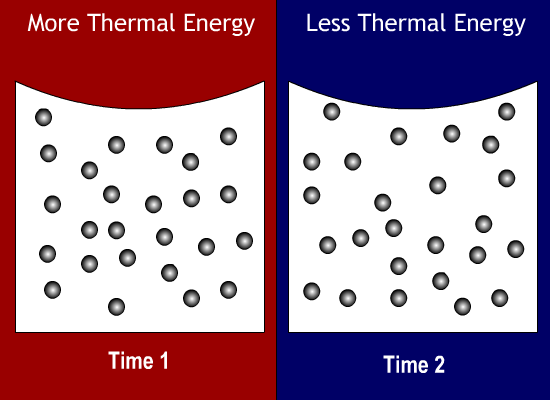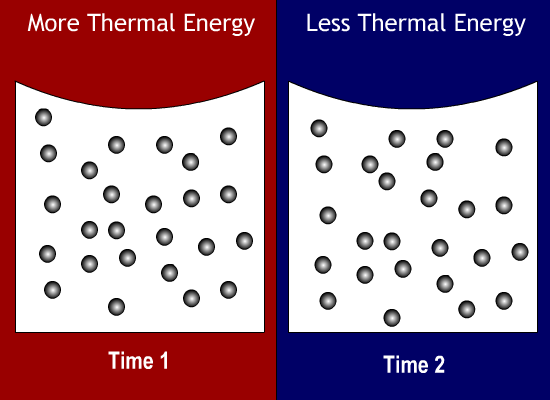
Thermal Energy Molecules
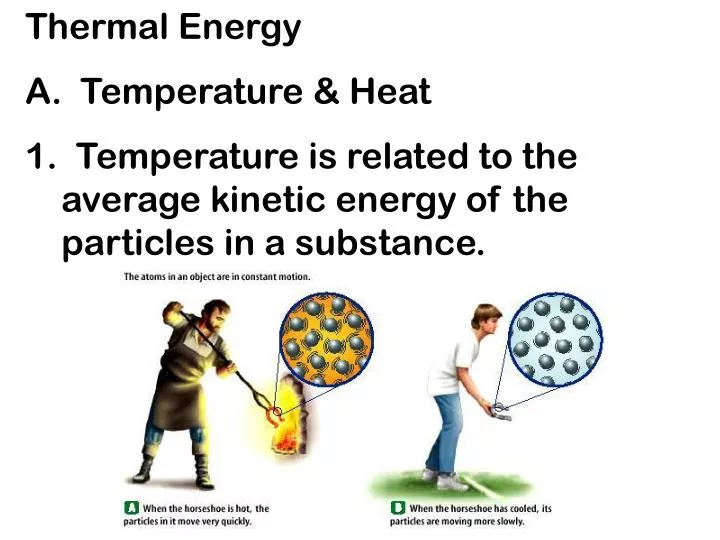
PPT Thermal Energy A Temperature Heat PowerPoint Presentation ID

PPT Thermal Energy A Temperature Heat PowerPoint Presentation ID

The 2 Types And 9 Forms Of Energy Kinetic And Potential

Energy Kinetic Energy Potential Energy Gravitational Potential Energy

Kinetic Energy Worksheet
What Makes Thermal Energy Kinetic - Thermal energy is the kinetic energy associated with the random motion of atoms and molecules Temperature is a quantitative measure of hot or cold When the atoms and molecules in an