Which Functional Group Has Acidic Properties The eponymous member of this grouping is the carboxylic acid functional group in which the carbonyl is bonded to a hydroxyl OH group As the name implies carboxylic acids are acidic meaning that they are readily deprotonated to form the conjugate base form called a carboxylate much more about carboxylic acids in Chapter 20
In an aqueous solution the functional group with acidic properties is the carboxylic acid group represented as C 0 C OH Carboxylic acids can donate a proton H resulting in the formation of a carboxylate ion and a hydronium ion which showcases their acidic behavior There are two key features of an acidic functional group the presence of a hydrogen atom that can dissociate from the group H and the ability of the remaining atoms to delocalize the resulting negative charge via resonance
Which Functional Group Has Acidic Properties
:max_bytes(150000):strip_icc()/common-acids-and-chemical-structures-603645_FINAL-54e6b0b3351b49dbb6cef54fd4817404.png)
Which Functional Group Has Acidic Properties
https://www.thoughtco.com/thmb/jdhnWsXq15TAcw2ZMutj6MCRMqU=/1500x0/filters:no_upscale():max_bytes(150000):strip_icc()/common-acids-and-chemical-structures-603645_FINAL-54e6b0b3351b49dbb6cef54fd4817404.png
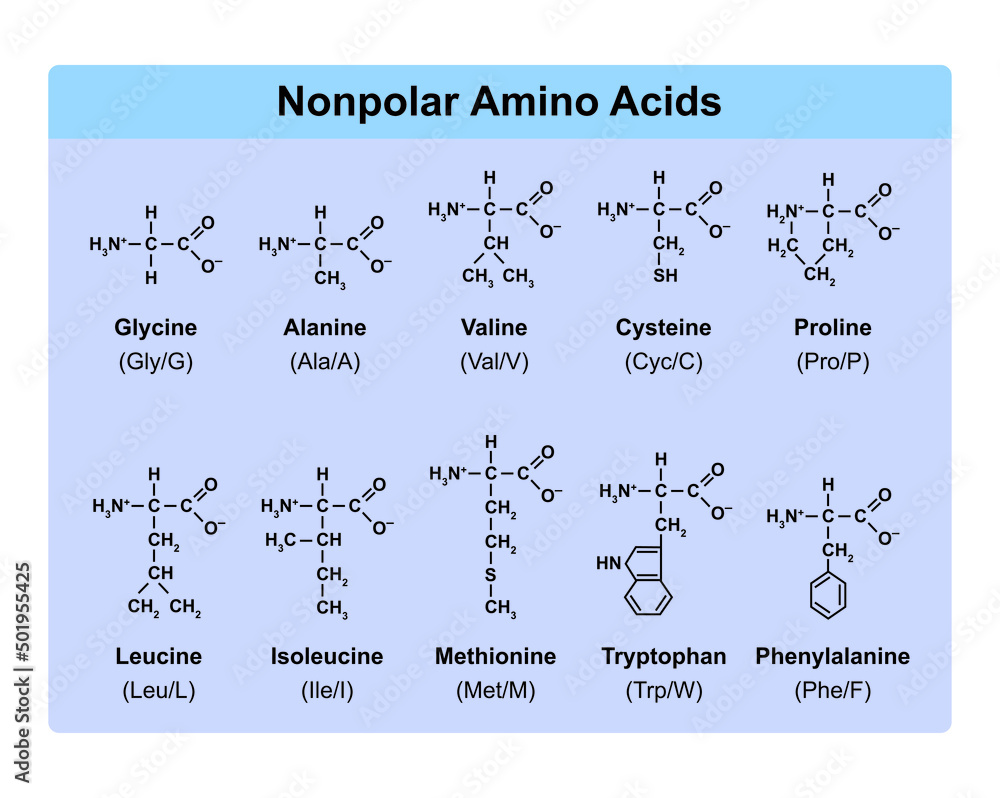
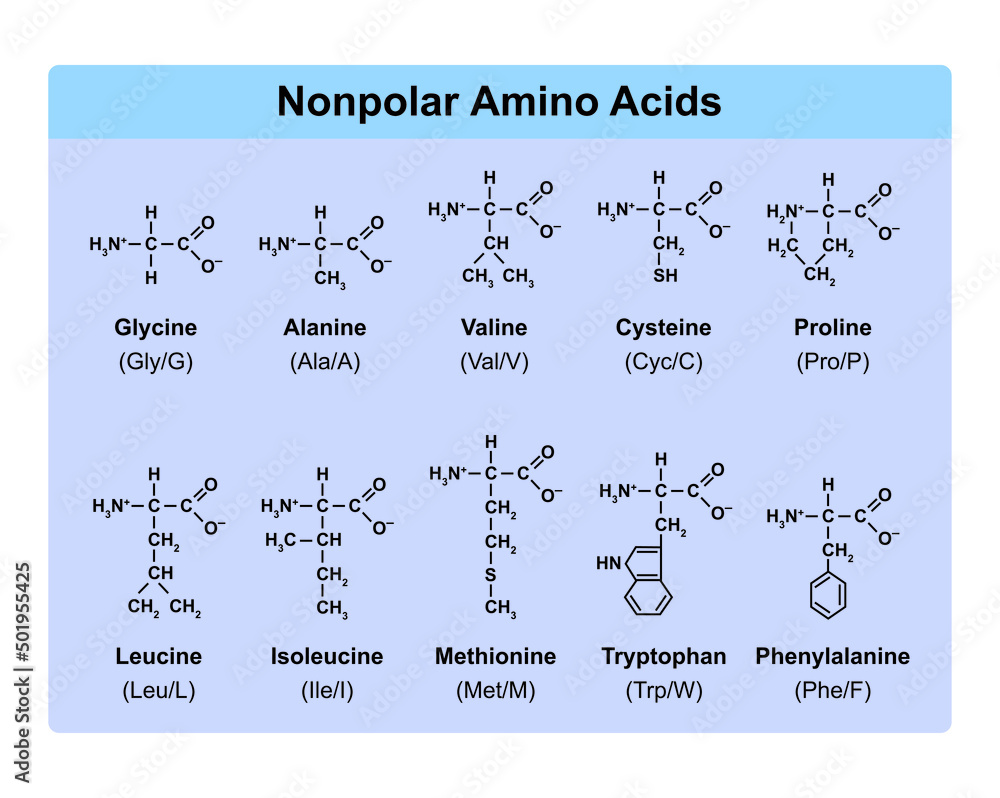
Amino Acids Types Table Showing The Chemical Structure Of Nonpolar
https://as1.ftcdn.net/v2/jpg/05/01/95/54/1000_F_501955425_mRwq71sDnTPAHXZRYgZqKNm2Em0G3fG5.jpg
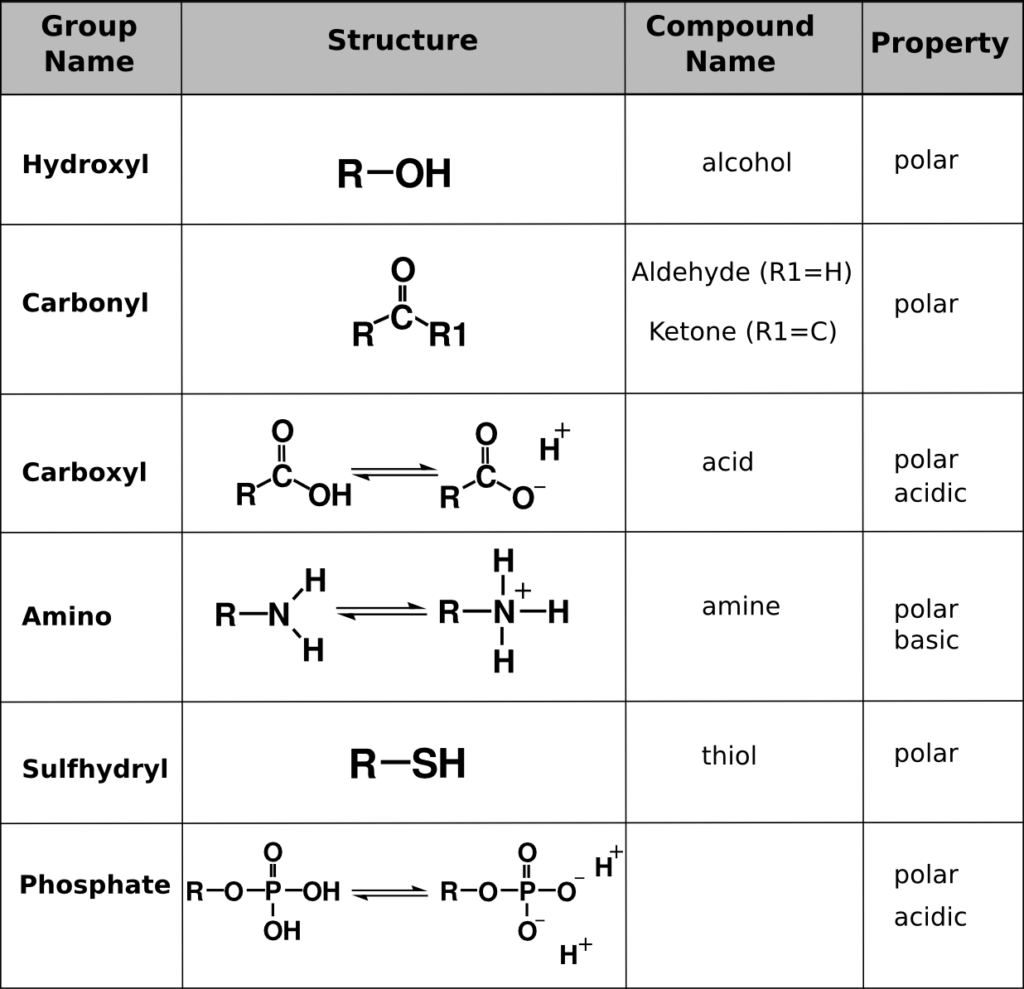
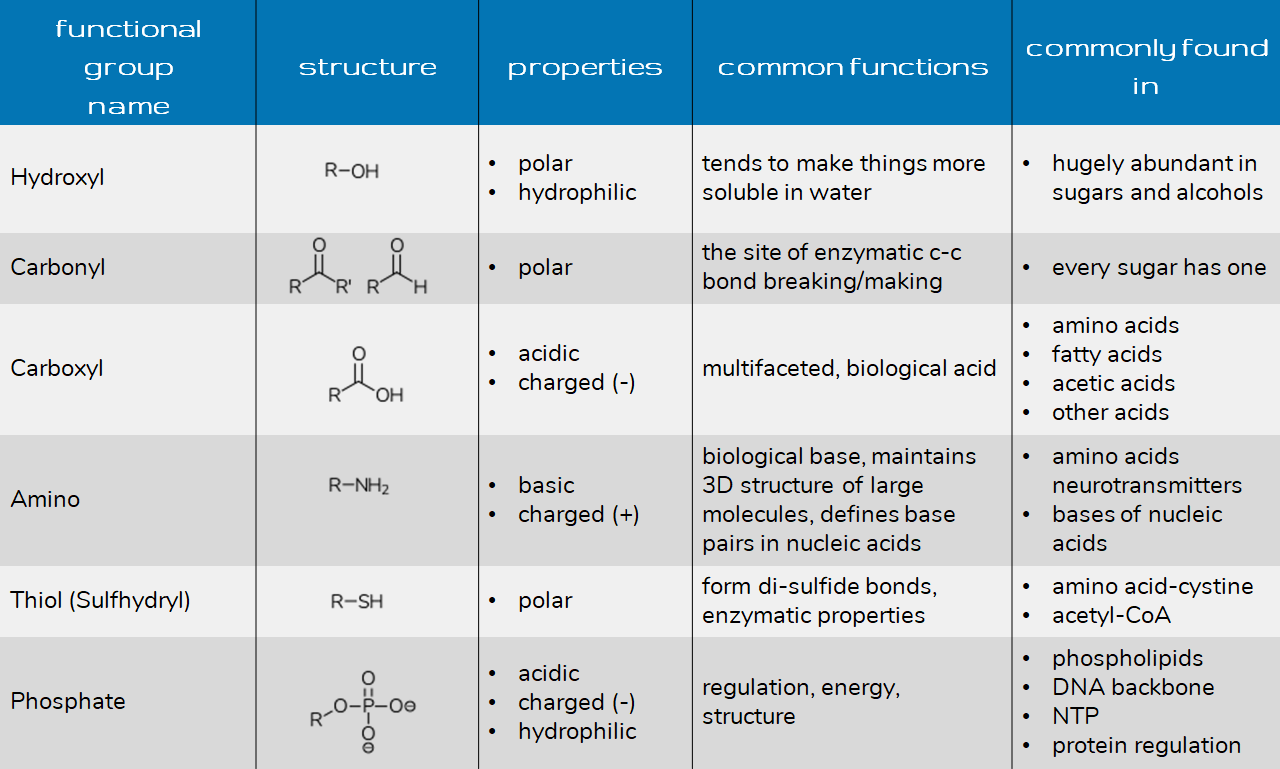
Functional Groups Biology 1101 Course Hub
https://openlab.citytech.cuny.edu/bio1101coursehub/files/2022/06/functional-1024x989.png
We have learned that different functional groups have different strengths in terms of acidity In this section we will gain an understanding of the fundamental reasons behind this which is why one group is more acidic than the other one The acidic functional group among the options provided is the carboxyl group The carboxyl group has the structure CO H and is characteristic of carboxylic acids It is acidic because it can donate a proton H in solution making carboxylic acids behave as acids
Many pharmaceutical molecules contain the carboxylic acid COOH functional group which exhibits acidic behavior in aqueous environments For an example consider the salicylic acid molecule shown at right For example which of these functional groups are acidic and which are basic hydroxyl carbonyl carboxyl amino sulfhydryl phosphate Hint Functional groups are smaller groups of atoms that are known to display a characteristic reactivity They are known to show a characteristic chemical behavior
More picture related to Which Functional Group Has Acidic Properties

OneClass Which Of The Following Compounds Is Most Acidic A I B II C
https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/110/11033032.png
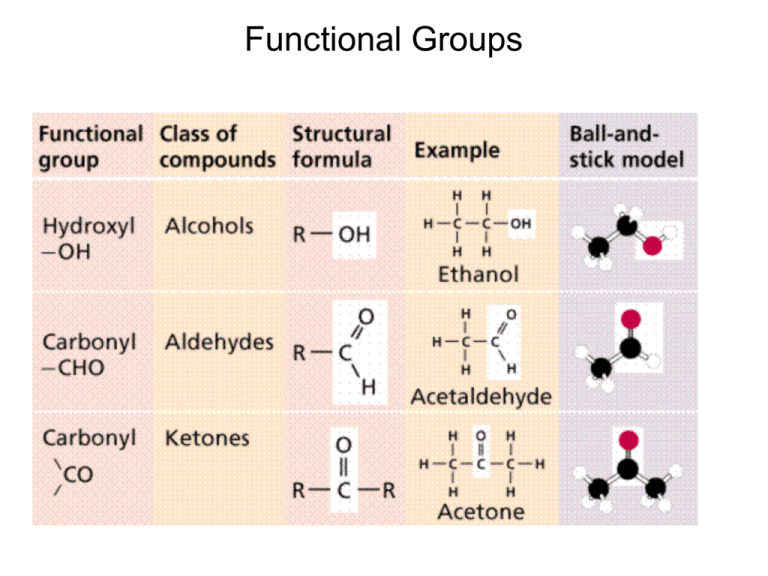
Functional Groups
https://s2.studylib.net/store/data/010079560_1-e718c9ee4d98cd7f5623399a1a0c88e0-768x994.png
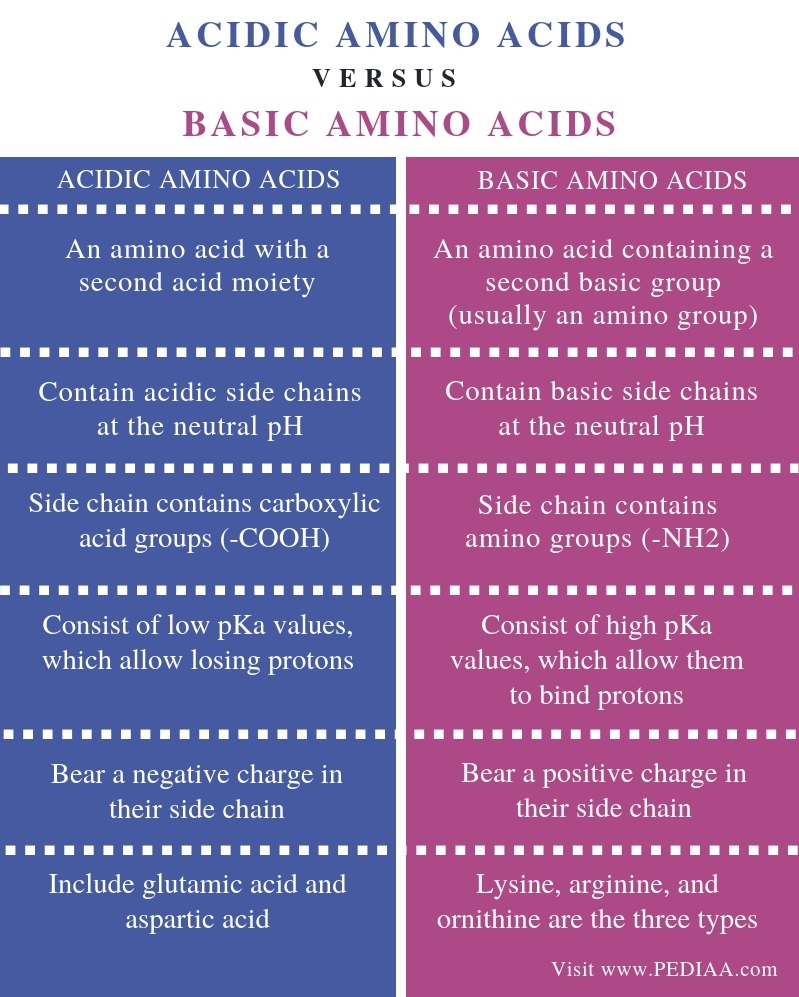
What Is The Difference Between Acidic And Basic Amino Acids Pediaa Com
http://pediaa.com/wp-content/uploads/2018/12/Difference-Between-Acidic-and-Basic-Amino-Acids-Comparison-Summary.jpg
Key functional groups include methyl nonpolar hydroxyl polar forms H bonds carboxyl acidic amino weak base sulfhydryl stabilizes proteins and phosphate found in nucleic acids Understanding these groups is crucial for Study with Quizlet and memorize flashcards containing terms like Hydroxyl Group OH Structure Hydroxyl Group Name of Compound Hydroxyl Group Function Functional Properties and more
[desc-10] [desc-11]

Amino Acids And Functional Groups General Biology Class Notes
https://bio16mit.files.wordpress.com/2013/07/aminoacids01.jpg
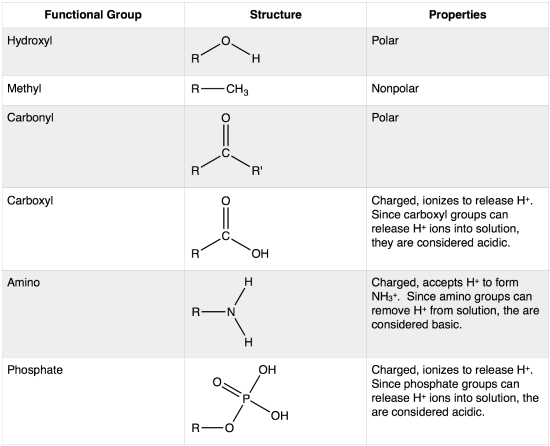
W2017 Lecture 03 reading Biology LibreTexts
https://bio.libretexts.org/@api/deki/files/16569/functional_grp_table.png?revision=1&size=bestfit&width=550&height=445
:max_bytes(150000):strip_icc()/common-acids-and-chemical-structures-603645_FINAL-54e6b0b3351b49dbb6cef54fd4817404.png?w=186)
https://chem.libretexts.org › Bookshelves › Organic...
The eponymous member of this grouping is the carboxylic acid functional group in which the carbonyl is bonded to a hydroxyl OH group As the name implies carboxylic acids are acidic meaning that they are readily deprotonated to form the conjugate base form called a carboxylate much more about carboxylic acids in Chapter 20
https://brainly.com › question
In an aqueous solution the functional group with acidic properties is the carboxylic acid group represented as C 0 C OH Carboxylic acids can donate a proton H resulting in the formation of a carboxylate ion and a hydronium ion which showcases their acidic behavior
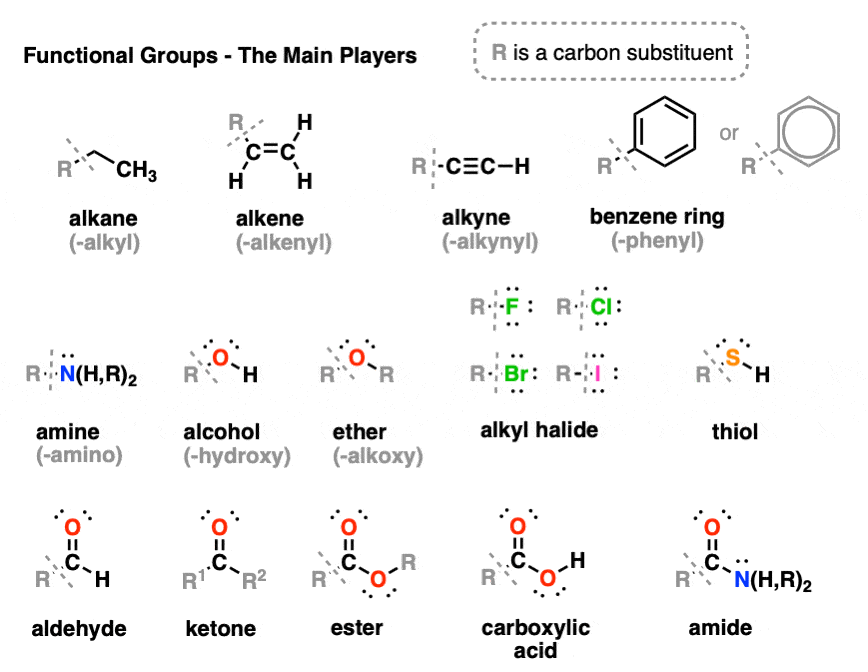
Functional Groups For Health And Bio Majors Chemistry Help Center

Amino Acids And Functional Groups General Biology Class Notes
Ester Functional Group Examples
Solved Predict The Relative Boiling Points For The Following

2 3 Functional Groups Chemistry LibreTexts
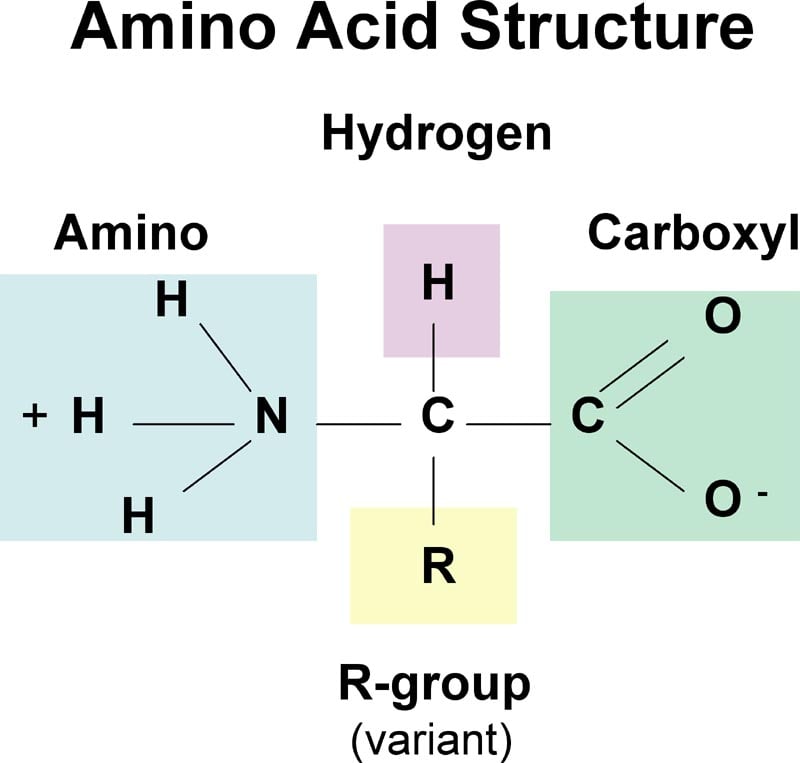
Amino Acids Properties Structure Classification Functions

Amino Acids Properties Structure Classification Functions

Give The Structural Formula Of The Functional Groups In A Acetic Acid
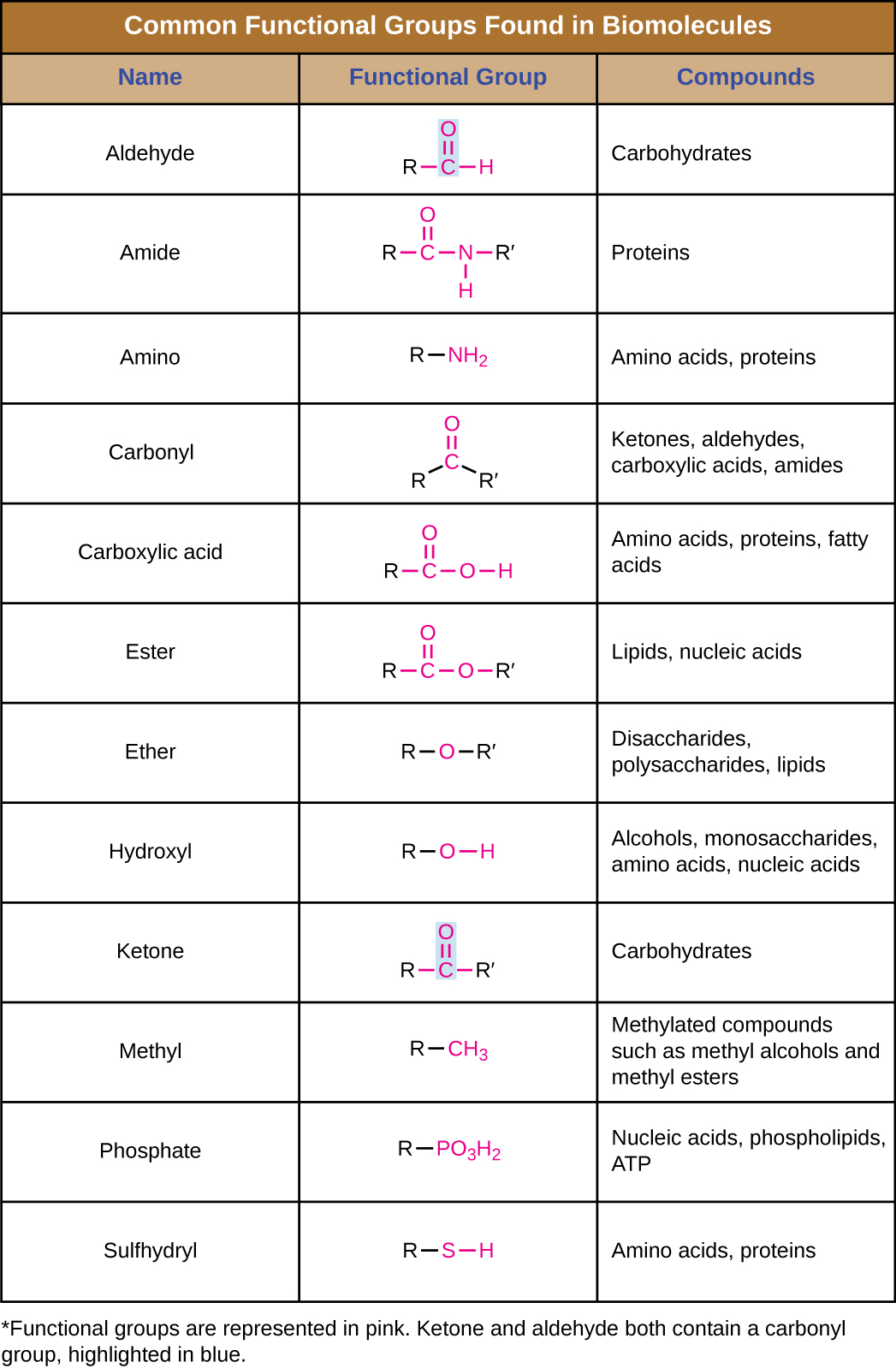
Organic Molecules Microbiology
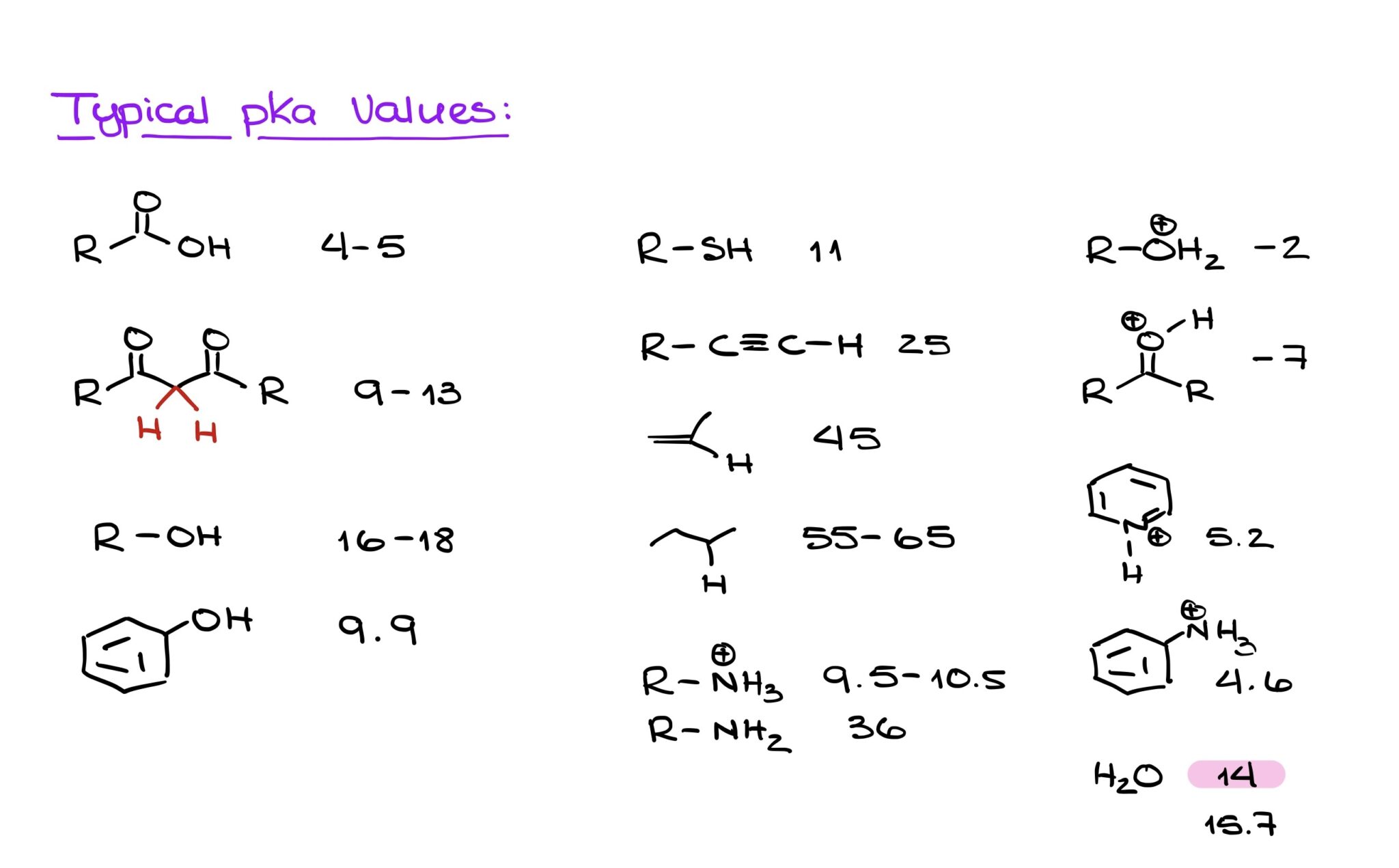
How To Find The Most Acidic Proton In A Molecule Organic Chemistry Tutor
Which Functional Group Has Acidic Properties - For example which of these functional groups are acidic and which are basic hydroxyl carbonyl carboxyl amino sulfhydryl phosphate Hint Functional groups are smaller groups of atoms that are known to display a characteristic reactivity They are known to show a characteristic chemical behavior